Alveolar echinococcosis in Switzerland
Authors:
Prof. Solange Bresson-Hadni, MD, PhD
Service de Médecine Tropicale et Humanitaire et
Service de Gastroentérologie et Hépatologie
Hôpitaux universitaires de Genève
Swiss Echinococcosis Network
E-Mail: solange.bresson-hadni@hcuge.ch
Sie sind bereits registriert?
Loggen Sie sich mit Ihrem Universimed-Benutzerkonto ein:
Sie sind noch nicht registriert?
Registrieren Sie sich jetzt kostenlos auf universimed.com und erhalten Sie Zugang zu allen Artikeln, bewerten Sie Inhalte und speichern Sie interessante Beiträge in Ihrem persönlichen Bereich
zum späteren Lesen. Ihre Registrierung ist für alle Unversimed-Portale gültig. (inkl. allgemeineplus.at & med-Diplom.at)
Alveolar echinococcosis (AE), a hepatic disorder caused by the continuous proliferation of the Echinococcus multilocularis metacestode (larvae), is one of the most potentially dangerous parasitic zoonosis in Europe. Its proliferative progression is very comparable to a slow growing liver cancer and, if left untreated, the 10-year mortality rate can reach up to 90%. Although still a rare disease, cases are increasing in the endemic area of Central Europe. Moreover, the geographical distribution of the parasite and the occurrence of human AE cases is expanding to countries previously considered AE free. After a short review of the current epidemiological situation of AE worldwide and its current presentation and management modalities in Western countries, we will focus on the situation in Switzerland.
Keypoints
-
Alveolar echinococcosis, caused by the slow intrahepatic development of the larval stage of Echinococcus multilocularis, is one of the most potentially dangerous zoonosis in Europe.
-
A multidisciplinary clinical-biological network, the Swiss Echinococcosis Network, initiated in 2020, is being implemented.
-
Preliminary data suggest that the steadily increasing annual incidence of the disease in Switzerland was probably underestimated and may be between 0.6 and 1.3 per 100,000, making Switzerland one of the highest risk countries in Europe.
-
The development of a national alveolar echinococcosis registry and reporting of cases would contribute to a better assessment of the situation.
Parasitic cycle and current epidemiological data
The main definitive hosts are foxes, less commonly dogs and other canids, harboring small (few millimeters in length) adult stage tapeworms in their small intestine. Parasite eggs are disseminated into the environment via feces. Natural intermediate hosts harboring larval stages in their liver are small rodents. Infection of these hosts occurs upon ingestion of infectious Echinococcus multilocularis eggs, which hatch in the intestine to release oncospheres that pass through the portal and lymphatic vessels to reach the liver, where they usually settle and develop as metacestodes (larvae). Humans are accidental “intermediate” hosts and a dead end for the parasitic life cycle. Infection of humans seems to occur mainly by ingestion of contaminated raw vegetables, wild berries, wild plants or by direct contact with an infected definitive host (Fig.1). Echinococcus multilocularis eggs can remain infective for months and up to a year, depending on environmental conditions. The eggs are sensitive to dehydration and heat but can survive freezing at –20°C.1 Anatomically, the parasitic lesion in the liver is an infiltrative mass consisting of multiple alveoli (Fig. 2). Microscopically, Echinococcus multilocularis produces alveolar microcysts that grow by exogenous proliferation. Distant metastases via hematogenous spread of viable metacestode fragments are possible, affecting mainly the lungs, more rarely the brain or other organs. In humans, the slow development of the metacestode is accompanied by an intense granulomatous reaction, which, with time, can develop into extensive fibrosis leading to obstructive complications. Metacestodes may also become inactive in some cases.
Fig. 1: Parasitic cycle of Echinococcus multilocularis (Source: Centers For Disease Control and Prevention [CDC]: https://www.cdc.gov/dpdx/echinococcosis/modules/Echinococcus_gran_LifeCycle_lg.jpg)
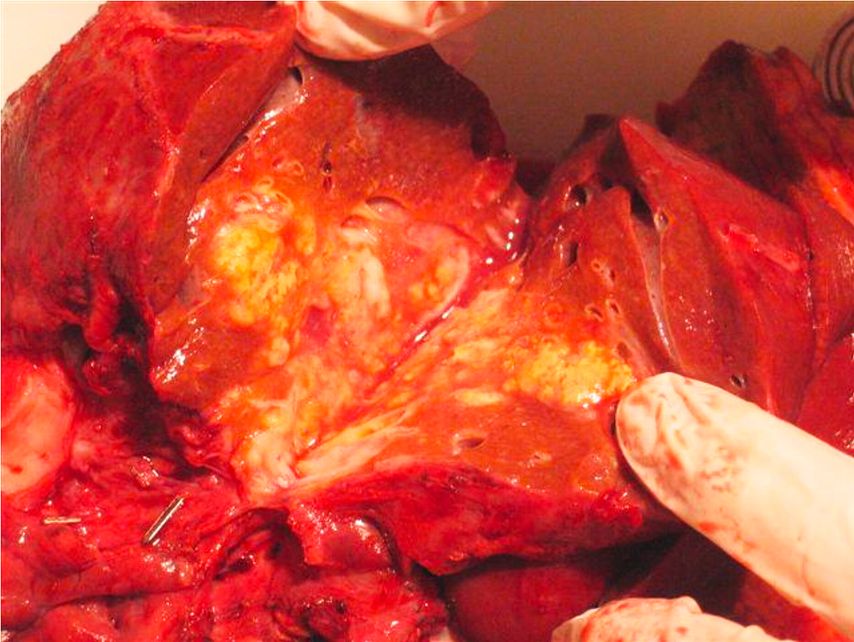
Fig. 2: Macroscopic cross-sectional view of an alveolar echinococcosis liver lesion after surgical resection. Dense fibrosis in the center and multiple parasitic alveolar structures at the periphery. No clear delimitation with the adjacent liver parenchyma
The disease is present only in the Northern Hemisphere. In 2010, 18451 new AE cases were estimated, 91% of them occurring in China, but the true number is expected to be higher, due to under-reporting in many endemic countries.1 In Europe, the main classical AE foci are located in Southern Germany, Switzerland, Western Austria and Eastern France. The prevalence of Echinococcus multilocularis in definitive and intermediate hosts increased markedly in the first 15 years of the 21st century,1,2 with a far broader geographic distribution of fox infections than earlier reported. Foxes also stray into urban areas, resulting in AE no longer being a rural disease only. Data from the French national registry FrancEchino, established in 1982, clearly indicated an increase in the annual number of newly reported cases: from around 15 patients until 2007 to 30 patients thereafter. Moreover, human cases are now detected in European countries where the disease had never been recognized, for example in the Baltic countries.1–3 In endemic areas, the annual AE incidence ranges from 0.03 to 1.2 per 100,000. In North America, Northwestern Alaska has long been an endemic area for Echinococcus multilocularis with human cases reported in Inuit communities. More recently, human cases were reported in Canada4 and now also elsewhere in the USA.5
Clinical presentation
In immunocompetent patients, where the metacestode grows very slowly, symptoms can arise after a latent period of 10 to 15 years. Early symptoms are mostly non-specific including fatigue and abdominal pain.1,6 In more advanced cases, pseudoneoplastic hepatomegaly, contrasting with a preserved general status, liver abscess due to bacterial superinfection of a central necrotic area, cholestatic jaundice or cholangitis may reveal the disease. In European countries, AE is nowadays more often diagnosed at an earlier (often asymptomatic) stage than in the past, following ultrasonography or CT-scan of the liver performed for other purposes. Recent data from the FrancEchino registry indicate that 60% of AE patients diagnosed from 2014 to 2019 had no symptoms at diagnosis,7 whereas during the 1982–1992 period, nearly 80% were symptomatic, half of whom presented with jaundice, a hallmark of advanced AE.8 The Zurich Echinococcosis cohort study recently reported similar results: from the 151 immunocompetent AE patients, 56% were asymptomatic at diagnosis.9 Hematogenous spread from supra-hepatic veins to distant organs may cause the initial symptom of AE,1,10 lung localizations being the most frequent and revealing AE in about 5% of the cases. Distant parasitic brain involvement is the revealing manifestation in only 1% of the cases in European countries, whereas it is more frequent in China.1
A few years ago, data from FrancEchino indicated an increased number of AE cases in the context of immunodeficiency.11 These are mainly patients with solid cancers, chronic inflammatory diseases, autoimmune diseases and, with a lower incidence, malignant hematological disorders, recipients of solid-organ or bone marrow transplantations and patients with HIV/AIDS. This now concerns 25% of incidental AE cases in France.7 More recently, two Swiss centers in charge of AE patients care reported similar observations.9,12 Interestingly, AE in this particular situation is generally discovered by chance during radiological follow-up of the underlying conditions treated with immunosuppressive drugs. In the most recent series from Zurich, nearly 80% of these patients were asymptomatic at the time of diagnosis.9 Immunosuppressive therapy may accelerate the growth of parasitic lesions, shortening the time interval between infection and diagnosis. Another hypothesis could be a reactivation of latent microscopic AE liver lesions that were controlled by the host’s immune defenses until then.13
Diagnosis
Fig. 3: Imaging aspect of alveolar echinococcosis liver lesion. Abdominal ultrasonography. Hepatic alveolar echinococcosis located in the right liver, segment VI: ill-delimited heterogeneous lesion with mixed echogenicity related to calcified and cystic areas
Diagnosis of AE is multimodal, based on clinical presentation along with epidemiological data, typical imaging features and serological findings. Liver ultrasonography (US) is one of the initial key examinations (Fig. 3). Images are typical in most cases, showing heterogeneous, mostly hyperechogenic lesions with irregular and ill-defined borders. Scattered calcifications are very common. Color Doppler sonography fails to detect a vascular flow in the solid components of the lesion. US may also identify vascular and/or biliary involvement. However, radiologists must be aware of atypical ultrasonic aspects, which account for approximately 30% of the cases, particularly in the form of small liver nodules, which may be either single or multiple. These are increasingly described in asymptomatic patients in whom abdominal US is performed for a different indication. They could reflect early-stage AE, but putatively also be proliferatively inactive or died-out. In the latter, the small liver nodule, mostly massively calcified, remains stable during follow-up and does not require treatment. Additional, more specific imaging examinations are usually required (Fig. 4A–C). Computed tomography (CT) is helpful, particularly for heavily calcified lesions, as it also allows to detect biliary and vascular involvement (Fig.4A). Magnetic resonance imaging (MRI) may be particularly helpful in diagnosing atypical AE lesions; T2-weighted sequences can reveal the characteristic arrangement of the multiple metacestode microcysts resembling a “honeycomb-like” pattern (Fig. 4B).1,2,14 Furthermore, one typical radiological feature is the absence of contrast enhancement around the lesion on intravenous CT and MRI sequences, in contrast with intrahepatic cholangiocarcinoma, which is the most important differential diagnosis.
Fig. 4: Imaging aspect of alveolar echinococcosis liver lesion. Second-line radiological exams performed on the same patient and analyzing a lesion located in the right liver, segments IVa, V and VIII. A:injected computed tomography, post-contrast venous phase. Ill-delimited lesion with heterogeneous content. Necrotic central area, surrounded by fibrous tissue and small cystic areas at the periphery. Close contact with the right portal vein (PV). Punctiform calcification (arrow). B: Magnetic resonance imaging T2-weighted image showing several hyper-T2 microcysts (arrows) typical of alveolar echinococcosis and testifying to an active lesion. C: 18F-fluorodesoxyglucose (FDG)-positron emission tomography, early acquisition image (1 hour after FDG injection). Very active lesion with strong FDG uptake around the parasitic mass
Specific serology is a second step exam for suspect clinical cases.1 Currently, the sero-diagnostic strategy includes primary screening and one or more subsequent confirmatory test(s). In Switzerland, first-line tests generally consist of EgHF-ELISA using Echinococcus granulosus hydatid fluid antigen that allows primary immunodiagnosis for both AE and cystic echinococcosis with a 95% diagnostic sensitivity for AE. For subsequent specification of this anti-Echinococcus antibody response, Echinococcus multilocularis-specific ELISAs, using the Em2-antigen (94% specificity) and the reEm18-antigen (94% specificity) are frequently used.1,15 The confirmation test includes also an Echinococcus multilocularis immunoblotting that contributes significantly to the increase of diagnostic sensitivity.15 In combination with the Em2- and rec-Em18-ELISA, this immunoblot method allows diagnosis of both clinical and sub-clinical (including abortive) AE, with a sensitivity of nearly 100%.1,15
Percutaneous guided biopsy of AE lesions is not systematically performed, but may be useful in situations of atypical images and/or negative serology particularly in immunosuppressed patients, or in case of an exclusively extra-hepatic localization.1 Conventional pathological examination may not be conclusive in the presence of necrosis or non-specific granuloma. Immunohistochemistry with the monoclonal antibodies mAbEmG11 and mAbEmG3 may also be a useful tool for AE diagnosis.16,17 Moreover, reserving frozen tissue is of major importance to carry out E. multilocularis DNA detection by quantitative and/or nested PCR assays.1,2,18
A morpho-functional evaluation of the lesions by [18F]-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) at the time of diagnosis is a useful adjunct. It allows an indirect evaluation of the metacestode activity by revealing a hyperconsumption of FDG at the periphery of the parasitic lesion, within the periparasitic granuloma with a similar yield compared to MRI (Fig.4C).19 It also makes it possible to identify other parasitic intra- or extra-abdominal foci. However, for an optimal evaluation, it is essential to add an additional later measurement, i.e. 3 hours after FDG injection, to the measurement usually made at 1 hour post-injection.19,20
Treatment
A multidisciplinary and personalized approach is key to optimize the treatment of AE. Albendazole (ABZ) medication and surgery are the cornerstones of management. ABZ acts as parasitostatic on Echinococcus multilocularis metacestode. A partial liver resection aiming at a complete microscopic removal of the parasitic tissue, surrounded by ABZ intake remains the gold standard treatment in AE.20–23 ABZ is usually administered for a few months before resection and it is recommended to continue for 2 years after surgery.20,21 This approach is nowadays feasible in around 60% of cases in Europe, due to earlier diagnosis and progress in hepato-biliary surgery.22 In case of an unresectable lesion, ABZ is indicated for life, sometimes in association with instrumental procedures (e.g. per-cutaneous drainage of centro-parasitic abscess or interventional biliary endoscopy in case of parasitic biliary obstruction).24 AE patients referred for long-term, mostly life-long ABZ treatment, benefit from surveillance of the evolution of parasite viability indicators (Em18-ELISA and sequential 18F-FDG-PET).19,20 Also, therapeutic drug monitoring allows dosage adjustments based on ABZ-sulfoxide (the active metabolite) plasmatic levels, to optimize therapeutic efficacy and avoid liver and bone marrow toxicity.25 Liver transplantation (LT) may be discussed for end-stage symptomatic AE.1,2,26 Fortunately, indications for LT are nowadays very exceptional in Europe due to the less severe extent of AE at time of diagnosis than previously. A large series of patients with end-stage AE treated by ex vivo liver resection and auto-transplantation was recently published by the Chinese team from Urumqi.27 This approach seems attractive, as (unlike LT) it avoids the use of immunosuppressive drugs that can promote the growth of possible parasitic remnants. However, there was an overall mortality rate of 12% and no comparison was made with the alternative option of long-term ABZ therapy. With this medical approach, European centers currently report excellent survival rates of 90% at 5 years.28 In these countries, the prognosis of AE has improved considerably over the last 40 years, resulting in a nearly normal life-expectancy.6,28–30
Current situation in Switzerland
In Switzerland, the overall yearly AE incidence (AE cases/105 inhabitants) increased significantly from 0.1 (1993–2000) to 0.26 (2001–2005),31,32 ten times higher than that published by the French AE register. This higher incidence, observed since 2000, was preceded several years earlier by an increased fox population.32 More recent publications from two Swiss centers involved in the management of AE patients confirmed the observations previously made in France and in Germany by highlighting an increased number of both overall AE cases and AE in immunocompromised patients.9,12
The Swiss Echinococcosis Network was established in October 2020. It currently brings together clinicians from various backgrounds and specialties, as well as radiologists and biologists involved in AE care and diagnosis. Currently, 9 centers from 8 Swiss cantons are involved (Tab. 1), and additional centers have been contacted. The idea is to cover the whole country, including areas where AE has not – or not yet – been reported.
Tab. 1: Preliminary results from the 9 centers of the Swiss Echinococcosis Network: number of alveolar echinococcosis (AE) patients currently under follow-up and newly diagnosed cases (incident AE) in the last 2 years (2020–2021)
We performed an explorative survey by contacting all nine centers and collecting the number of AE patients currently under follow-up and newly diagnosed (incident AE cases) in 2020 and 2021. Results are presented in Table 1. The centers located in Bern and Zurich report the greatest number of cases. The proportion of AE patients having an immunosuppression-associated condition (IAC) varied from 2 to 31%, probably because of differences in the definition of IAC between centers. These very preliminary results allow us to estimate the number of patients currently being followed for AE in Switzerland to at least 586. In addition, this clinical inquiry allowed to identify at least 102 newly diagnosed AE cases over the last 2 years. This data needs to be refined, as cases are likely to be followed in other centers, including the private sector, and as duplicates have not been excluded.
We also obtained information from diagnostic laboratories specialized in echinococcosis serology. So far, we questioned the three laboratories most frequently involved in AE serodiagnosis, located in Basel, Bern and Zurich. We asked for the number of “new”Echinococcus multilocularis positive serological tests during the years of 2020 and 2021, by focusing on requests for Swiss residents. Results are summarized in Figure 5. Again, these are very preliminary results. Nevertheless, they indicated, for the year of 2020, a total of 94 “new” positive serodiagnoses for AE to which 4 additional cases were diagnosed by tissue Echinococcus multilocularis PCR without serology. However, as some of these cases may have been diagnosed in parallel in more than one laboratory, these data may be rather overestimating reality. In addition to these 94 incident serological AE cases, there are probably other cases for which serology yielded “only” Echinococcus spp. as a result (Fig. 5). Nevertheless, further work is needed to better qualify these data. The diagnostic laboratories were also able to provide data for 2021, showing a clear progression of “new”Echinococcus multilocularis positive serological results (n=138) (Fig. 5).
Fig. 5: Number (N) of “new” Echinococcus multilocularis (E.m) positive serological tests (Swiss patients only) in the last 2 years (2020–2021). Data obtained from the 3 microbiology laboratories most involved in alveolar echinococcosis sero-diagnosis in Switzerland
In summary, the clinical investigation (restricted to the participating centers in the network) suggested an average diagnosis of 51 new AE cases per year during the last two years, while the serological survey, which concerned a larger area, suggested a number nearly twice as high. Therefore, the current estimated annual incidence of AE in Switzerland could range from 0.58/105 to 1.33/105, as such significantly higher than previously published data. These preliminary results, which must be refined, would make Switzerland among the countries in Europe with the highest risk for AE.
Currently, the network meets semi-annually. It shares discussion on the management of complex AE cases. There are also invited lectures on different aspects of the disease and a project to prepare jointly, under the aegis of various learned societies, easily accessible AE treatment recommendations for Swiss practitioners. A reflection is also engaged on the implementation of collaborative studies together with the development of information to the public and other preventive actions. In fact, a recent survey of public knowledge about Echinococcus multilocularis in four European countries suggested that Switzerland had the highest level of knowledge as compared to other surveyed countries (Czech Republic, France and Germany). However, Switzerland showed the lowest level of perceived severity of the disease.33 In addition, given the current increasing number of incident AE cases, the development of a national AE registry should be a priority for the upcoming years. Finally, the Swiss Echinococcosis Network supports the introduction of AE in the list of notifiable diseases of the Swiss Federal Office of Public Health and also strongly supports a “OneHealth” approach to tackle this problem from both medical and veterinary point of view.
Acknowledgments:
Prof S. Bresson-Hadni is very grateful to Profs François Chappuis and Bruno Gottstein for their involvement in the initiation of the Swiss Echinococcosis Network, and to all members of the Network for reviewing and commenting the manuscript. She is also very grateful to Adèle Bresson for her very efficient technical assistance.
Literature:
1 Bresson-Hadni S et al.: Hepatic alveolar echinococcosis. Semin Liver Dis 2021; 41: 393-408 2 Wen H et al.: Echinococcosis: Advances in the 21st century. Clin Microbiol Rev 2019; 32: 1-39 3 Vuitton DA et al.: Clinical epidemiology of human AE in Europe. Vet Parasitol 2015; 213: 1110-20 4 Massolo A et al.: European Echinococcus multilocularis identified in patients in Canada. N Engl J Med 2019; 381: 384-5 5 Polish LB et al.: European haplotype of Echinococcus multilocularis in the United States. N Engl J Med 2022; 387: 1902-1904 6 Peter L et al.: Parasites of the liver – epidemiology, diagnosis and clinical management in the European context. J Hepatol 2021; 75: 202-18 7 National Reference Center for Echinococcoses: Annual activity report 2021; https://cnr-echinococcoses-ccoms.univ-fcomte.fr 8 Piarroux M et al.: Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. J Hepatol 2011; 55: 1025-33 9 Deibel A et al.: Characteristics and clinical course of alveolar echinococcosis in patients with immunosuppression-associated conditions: a retrospective cohort study. Pathogens 2022; 11: 441 10 Caire Nail L et al.: Disseminated alveolar echinococcosis resembling metastatic malignancy: a case report. J Med Case Rep 2017; 11: 113 11 Chauchet A et al.: Increased incidence and characteristics of alveolar echinococcosis in patients with immunosuppression-associated conditions. Clin Inf Dis 2014; 59: 1095-104 12 Lachenmayer A et al.: Elevated incidence of alveolar echinococcosis in immunocompromised patients. Food Waterborne Parasitol 2019: 16: e00060 13 Autier B et al.: Alveolar echinococcosis in immunocompromised hosts. Clin Microbiol Inf 2022; S1198-743X(22)00630-9 14 Kodama Y et al.: Alveolar echinococcosis: MR findings in the liver. Radiology 2003; 228: 172-7 15 Gottstein B et al.: Diagnostic and follow-up performance of serological tests for different forms/courses of alveolar echinococcosis. Food Waterborne Parasitol 2019; 16: e00055 16 Barth TFE et al.: Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis 2012; 6: e1877 17 Reinehr M et al.: Pathology of echinococcosis: A morphologic and immunohistochemical study on 138 specimens with focus on the differential diagnosis between cystic and alveolar echinococcosis. Am J Surg Pathol 2020; 44: 43-54 18 Knapp J et al.: Molecular diagnosis of alveolar echinococcosis in patients based on frozen and formalin-fixed paraffin-embedded tissue samples. Parasite 2022; 29: 4 19 Caoduro C et al.: The role of delayed 18F-FDG PET Imaging in the follow-up of patients with alveolar echinococcosis. J Nucl Med 2013; 54: 16 20 Deibel A et al.: Evaluation of a structured treatment discontinuation in patients with inoperable alveolar echinococcosis on long-term benzimidazole therapy: A retrospective cohort study. PLoS Negl Trop Dis 2022; 16: e0010146 21 Brunetti E et al.: Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114: 1-16 22 Joliat GR et al.: Outcomes after liver resection for hepatic alveolar echinococcosis: a single-center cohort study. World J Surg 2015; 39: 2529-34 23 Salm LA et al.: Surgical treatment strategies for hepatic alveolar echinococcosis. Food Waterborne Parasitol 2019; 15: e00050 24 Ambregna S et al.: A European survey of perendoscopic treatment of biliary complications in patients with alveolar echinococcosis. Expert Rev Anti Infect Ther 2017; 15: 79-88 25 Bresson-Hadni S et al.: Tobacco, cannabis, and liquorice: Hidden players altering albendazole metabolism in patients with hepatic alveolar echinococcosis. J Hepatol 2021; 74: 471-3 26 Koch S et al.: Experience of liver transplantation for incurable alveolar echinococcosis: a 45 case European collaborative report. Transplantation 2003; 75: 856-63 27 Aji T et al.: Ex vivo liver resection and autotransplantation as alternative to allotransplantation for end-stage hepatic alveolar echinococcosis. J Hepatol 2018; 69: 1037-46 28 Beldi G et al.: Is ex vivo liver resection and autotransplantation a valid alternative treatment for end-stage hepatic alveolar echinococcosis in Europe? J Hepatol 2019; 70: 1030-1 29 Torgerson PR et al.: Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J Hepatol 2008; 49: 72-7 30 Grüner B et al.: Comprehensive diagnosis and treatment of alveolar echinococcosis: a single-center long-term observational study of 312 patients in Germany. GMS Infect Dis 2017; 5: Doc01 31 Deplazes P et al.: Global distribution of alveolar and cystic echinococcosis. Advances in Parasitology 2017; 95: 315-49 32 Schweiger A et al.: Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis 2007; 13: 878-82 33 Hegglin D et al.: Survey of public knowledge about Echinococcus multilocularis in four European countries: Need for proactive information. BMC Public Health 2008; 8:24
The Swiss Echinococcosis Network
Co-coordinators:
Prof. Bruno Gottstein, Institute for Infectious Diseases, Immunoparasitology, Faculty of Medicine, University of Bern
Prof. François Chappuis and Prof. Solange Bresson-Hadni, Division of Tropical and Humanitarian Medicine, University Hospitals of Geneva (HUG) and University of Geneva
Members (alphabetical order):
Basel:
Prof. Markus Heim, Division of Gastroenterology and Hepatology, University Hospital Basel
PD Dr Tobias Heye, Clinic of Radiology and Nuclear Medicine, University Hospital Basel
PD Dr Andreas Neumayr, Swiss Tropical and Public Health Institute, Allschwil
Dr. Beatrice Nickel, Dr. Marie-Thérèse Ruf, and Maura Concu, Diagnostic Center, Swiss Tropical and Public Health Institute, Allschwil
Prof. Marcel Stoeckle, Department of Infectious Diseases, University Hospital Basel
Bern:
Prof. Guido Beldi, PD Dr Anja Lachenmayer, Prof. Daniel Candinas, Dr Severin Gloor, Department of Visceral Surgery and Medicine, Inselspital, University Hospital Bern and University of BernProf. Annalisa Berzigotti, Hepatology, Department of Visceral Surgery and Medicine, Inselspital, University Hospital Bern and University of BernProf. Britta Lundström-Stadelmann, Institute of Parasitology, Department of Infectious Diseases and Pathobiology, Vetsuisse FacultyDr. Alexander Oberli, Prof. Norbert Müller, Institute of Infectious Diseases, University of BernProf. Andri Rauch, PD Dr. Cornelia Staehelin, Department of Infectious Diseases, Inselspital, University Hospital Bern and University of Bern
Geneva:
Dr. Ilinca Constantinescu, Abdominal and Interventional Radiology Unit, Radiology Department, University Hospitals of Geneva (HUG) and University of Geneva
Prof. Laurent Spahr, Division of Gastroenterology and Hepatology, University Hospitals of Geneva (HUG) and University of Geneva
Prof. Christian Toso, Surgery Department, University Hospitals of Geneva (HUG) and University of Geneva
Lausanne:
Prof. Matthias Cavassini, Department of Infectious Diseases, University Hospital Lausanne (CHUV) and University of Lausanne (UNIL)
Dr. Christopher Doerig, Private Gastroenterology and Hepatology practice, Lausanne
Prof. Jean-François Dufour, Private Centre for Digestive Diseases, Lausanne
Prof. Nermin Halkic, Dr. Gaëtan-Romain Joliat, Department of Visceral Surgery, University Hospital Lausanne (CHUV) and University of Lausanne (UNIL)
Dr. Nicola Leggieri, Private Internal Medicine and Infectious Diseases practice, Gland
Prof. Darius Moradpour, Dr. Montserrat Fraga, Gastroenterology and Hepatology Department, University Hospital Lausanne (CHUV) and University of Lausanne (UNIL)
Prof. Sabine Schmidt, Prof. Alban Denys, Department of Diagnostic and Interventional Radiology, University Hospital Lausanne (CHUV) and University of Lausanne (UNIL)
Dr. Frédéric Tissot, Private Infectious Diseases practice, Lausanne
Neuchâtel:
Dr. Olivier Clerc, Infectious Diseases Unit, Neuchâtel Hospital
St. Gallen:
PD Dr. Dr. David Semela, Division of Gastroenterology and Hepatology, Kantonsspital St. Gallen
Dr. Carol Strahm, Division of Infectious Diseases, Kantonsspital St Gallen
Sion:
Prof. Stephane Emonet, Infectious Diseases Unit, Valais Hospital, Sion
Dr. Ian Fournier, Visceral Surgery Unit, Valais Hospital, Sion
Dr. Philippe Renard, PD PhD Dr. Christian Mottet, Gastroenterology and Hepatology Unit, Valais Hospital,Sion
Zurich:
Prof. Peter Deplazes, Dr. Felix Grimm, Dr. Philipp Andreas Kronenberg, Institute of Parasitology, University of Zurich
Prof. Beat Müllhaupt, Dr. Rudolf Ansgar Deibel, Department of Gastroenterology and Hepatology, University Hospital Zurich and University of Zurich
Prof. Achim Weber, Dr. Michael Reinehr, Department of Pathology and Molecular Pathology, University Hospital Zurich and University of Zurich
Das könnte Sie auch interessieren:
Das kardiovaskuläre Risiko von IBD-Patienten
Eine aktive IBD erhöht das Risiko für kardiovaskuläre Erkrankungen, während bestehende kardiovaskuläre Probleme die Wahl der Medikation erschweren. Das Ziel ist es, die richtige Balance ...
Aktuelle Studien aus Gastroenterologie und Hepatologie
Am Jahreskongress der Schweizerischen Gesellschaft für Gastroenterologie (SSG) und der Swiss Association for the Study of the Liver (SASL) vom 11. bis 12. September 2025 in Interlaken ...
Neues aus der Gastroenterologie
Nicht jede Alkoholisierung ist auf Alkoholkonsum zurückzuführen. Beim sogenannten Eigenbrauer-Syndrom kommt es infolge pathologischer Auffälligkeiten des Darmmikrobioms zur endogenen ...