Cornerstone of First-line Treatment in Non-oncogene Addicted Advanced NSCLC
Author:
Dr. Maurice Pérol
Léon Bérard Cancer Center
Lyon, France
E-Mail: maurice.perol@lyon.unicancer.fr
The role of cytotoxic chemotherapy in the first-line treatment of metastatic non-small cell lung cancer (NSCLC) has diminished with the advent of precision medicine and the superiority of tyrosine kinase inhibitors over first-line chemotherapy in the treatment of adenocarcinomas with oncogene addiction.1 The emergence of immunotherapy with immune checkpoints inhibitors (ICIs) becoming a new standard for second-line treatment2–5 has led to their development into the first-line strategy, resulting in a profound modification of the treatment algorithm despite the maintenance of cytotoxic chemotherapy in the majority of patients.
Lessons from the initial development of ICIs
Initial development involved patients previously treated with chemotherapy, demonstrating significant superiority to docetaxel for anti-programmed cell death protein 1 (PD-1; nivolumab, pembrolizumab) and atezolizumab (anti-PD-L1) in the 2nd or 3rd line of treatment, doubling overall survival at 2 years.2–5 Current evidence suggests that about 15% of patients have prolonged survival.6 Autoimmune toxicities occurring in about 20% of patients require specific management algorithms with rapid initiation of corticosteroid therapy in order to avoid progression to severe forms.7
The fact that only a minority of patients respond to anti-PD-1/PD-L1 underscores the need for identifying predictive biomarkers of response to ICIs. The level of PD-L1 expression by tumour cells correlates with the probability of response to anti-PD-1/PD-L1 and with the magnitude of the survival benefit of anti-PD(L)-1 monotherapy compared to docetaxel in second line.4 The lack of PD-L1 expression in approximately one-third of NSCLC does not preclude a response to treatment but is associated with a significant risk of early progression.3
ICIs development in 1st line treatment of NSCLC
Two main strategies have been explored to introduce anti-PD(L)-1 into the first-line treatment of metastatic NSCLC. The first was aimed at replacing chemotherapy, requiring the selection of patients with a sufficiently high probability of response to ICI to be superior to a platinum-based doublet. Two biomarkers were used in this context: the level of PD-L1 expression with cut-offs that vary according to the trials, and the mutational load. Anti-PD(L)-1 were assessed in NSCLC, either alone8–12 or in combination with anti-CTLA-4 12–15. The second strategy was based on the combination of anti-PD(L)-1 with cytotoxic chemotherapy, without the need for prior selection on the level of PD-L1 expression.16–22
Immunotherapy alone without chemotherapy
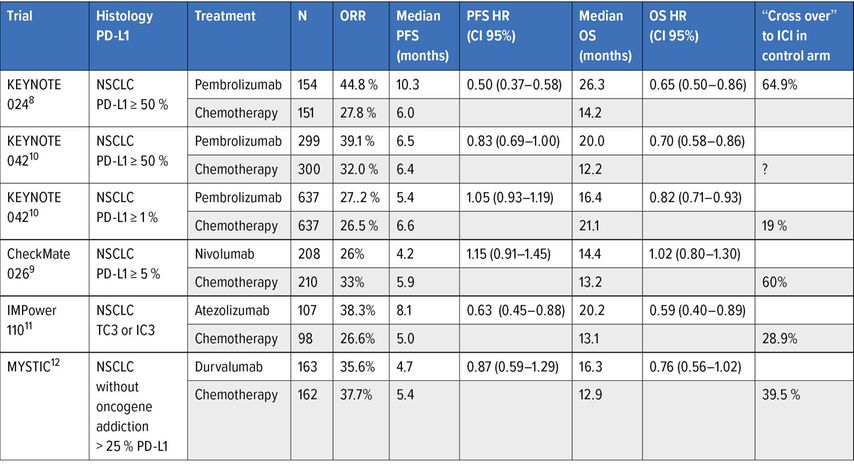
Comparison of anti-PD(L)-1 as single agent with chemotherapy (Table 1)
Table 1: Phase III trials assessing anti-PD(L)-1 as monotherapy in 1st line treatment of advanced NSCLC
The PD-L1 ≥50% of tumour cells cut-off for predicting a response to treatment was established in in the phase I of pembrolizumab and formed the basis of the Keynote 024 trial.8 This trial demonstrated a major benefit in terms of overall survival for pembrolizumab compared to first-line chemotherapy (median from 14,2 months to 26,3 months, HR: 0,65, 95% CI: 0,50–0,86) despite a crossover to immunotherapy for more than 60% of patients in the control arm.8 The significance of this cut-off for PD-L1 expression was confirmed by the negativity of the Checkmate 026 trial comparing nivolumab to first-line chemotherapy by selecting patients at a lower PD-L1 expression cut-off (≥5%)9, and the KEYNOTE 042 trial evaluating pembrolizumab against a platinum doublet in NSCLC expressing PD-L1 in at least 1% of tumour cells10. These data led to the first change in the treatment algorithm now based on the level of PD-L1 expression, considering pembrolizumab as the standard of care in patients eligible for immunotherapy with PD-L1 ≥50%. Anti-PD-L1 was also compared to first-line chemotherapy, also illustrating the impact of the level of PD-L1 expression in patient selection. Durvalumab showed no significant improvement in survival compared with chemotherapy in NSCLC with PD-L1 ≥25% of tumour cells12 whereas in the IMPower 110 trial, atezolizumab was superior to chemotherapy only in tumours expressing PD-L1 in more than 50% of tumour cells or 10% of accompanying immune cells11.
Comparison of anti-CTLA-4 + anti-PD(L)-1 combination with chemotherapy
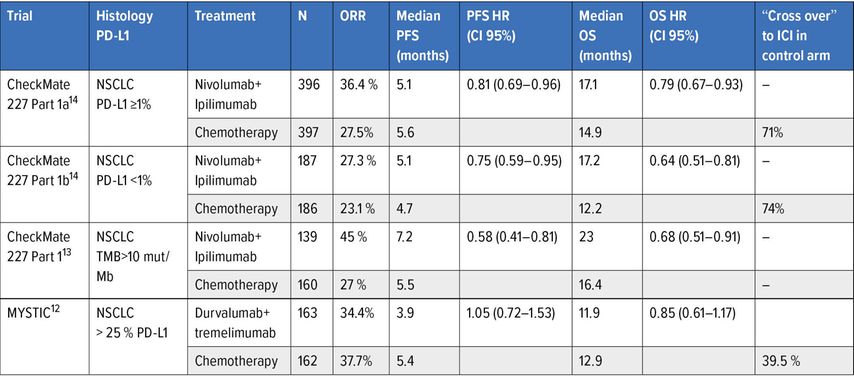
The ipilimumab-nivolumab combination was evaluated in comparison with chemotherapy in the first part of the CheckMate 227 trial, evaluating the predictive impact of two biomarkers, on the one hand a high mutational load with a cut-off of 10 mutations/megabase13, and on the other hand the expression of PD-L1 at the cut-off of ≥1% on overall survival14. The presence of a high mutational load was found to be predictive of a PFS benefit compared to chemotherapy, independently of PD-L1 expression13 but not of a survival benefit, which appeared independent of the level of the mutational load13. The other main co-criterion of judgement was also met with a significant survival benefit in ex-primary PD-L1 tumours in at least 1% of tumour cells (Table 2).14 The most noteworthy element is the prolonged duration of response with dual immunotherapy (median duration at 23,2 months), which accounts for the maintenance of the long-term survival benefit (33% versus 22% at 3 years).15 The other important element is that the survival benefit is of a similar magnitude for tumours not expressing PD-L1 in an exploratory analysis. It can therefore be concluded that neither the level of PD-L1 expression at the 1% threshold nor the mutation load are biomarkers predictive of the efficacy of the ipilimumab-nivolumab combination in terms of survival.
Table 2: Phase III trials evaluating combination of anti-CTLA-4 and anti-PD(L)-1 in 1st line treatment of advanced NSCLC
An alternative combination of anti-CTLA-4 + anti-PD-L1 with tremelimumab-durvalumab was evaluated in the MYSTIC study compared to first-line chemotherapy.12 The trial did not show a significant difference in overall survival in favour of the combination tremelimumab-durvalumab relative to chemotherapy for tumour expression of PD-L1 ≥25% (Table 2). Ahigh mutation load determined in circulating tumour DNA with cut-offs of 16 and 20 mutations per Mb retrospectively identified patients benefiting from this combination, which was not the case for durvalumab alone.12 However, prospective validation of the mutation load measured in circulating DNA at the 20 mutations/Mb threshold in the subsequent NEPTUNE trial failed.
It is therefore currently impossible to formally identify patients who may benefit from an anti-CTLA-4 + anti-PD(L)-1 combination, that generates a higher level of autoimmune-mediated toxicity than with anti-PD(L)-1 monotherapy. All of these data make it difficult to position this dual immunotherapy, which has been registered in the US for PD-L1 tumours ≥1% but is not approved in Europe.
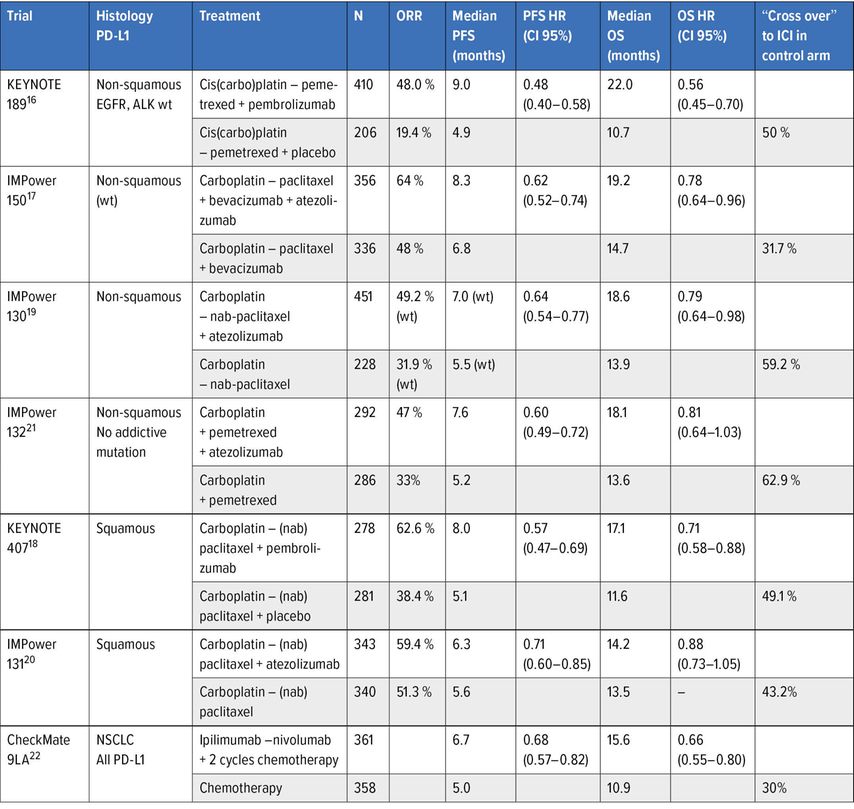
Combination of anti-PD(L)-1 with cytotoxic chemotherapy (Table 3)
Table 3: Phase III trials assessing combinations of ICIs and chemotherapy in frontline treatment of advanced NSCLC
The second avenue for the development of first-line anti-PD-1/PD-L1 was the combination of chemotherapy and anti-PD-1/PD-L1. The rationale for this combination is based on the ability of certain cytotoxic agents to generate an immune response by triggering immunogenic cell death and to modify the tumour immune microenvironment by reducing immunosuppressive cells and increasing the proportion of cytotoxic T lymphocytes.
A large phase III trial program evaluating several chemotherapy and anti-PD(L)-1 combinations has been conducted mainly separately in squamous cell and non-small cell cancers, the results of which are currently available and are shown in Table 3. Overall, the addition of anti-PD(L)-1 to a platinum-based doublet increases the response rate and PFS and generally improves survival, despite a significant crossover to immunotherapy for patients initially receiving chemotherapy alone; the magnitude of benefit no longer appears to be correlated with the level of PD-L1 expression in the most robust trials with pembrolizumab, with a persistent survival benefit for NSCLC expressing little or no PD-L1.16, 18 The impact of combination with chemotherapy prevents the excess of early progression consistently observed when immunotherapy is used alone. The duration of response is, however, shorter compared to immunotherapy alone, meaning that a proportion of responders are in fact only sensitive to chemotherapy. The toxicity of these combinations is the sum of the toxicities of each of their components, chemotherapy and immunotherapy, without any prohibitive element.16–21 No biomarkers have been shown to predict the effect of the addition of anti-PD(L)-1 to chemotherapy, such as the degree of PD-L1 expression16, 18 or the mutational load, partly because of the confounding role of chemotherapy in the treatment effect.
Association of cytotoxic chemotherapy with anti-CTLA-4 and anti-PD(L)-1
The combination of ipilimumab-nivolumab with chemotherapy was evaluated in comparison to chemotherapy in the CheckMate 9LA trial.22 The experimental arm limited chemotherapy to two cycles to address the problem of potential early progression under immunotherapy while limiting the cumulative toxicity associated with chemotherapy. This combination was superior to chemotherapy alone in terms of overall survival (median survival from 10,9 to 15,6 months, HR: 0.66 [0,55–0,80]), but also in terms of response, duration of response and progression-free survival.22 This benefit is of similar magnitude regardless of the level of PD-L1 expression. The tolerability of this triple combination is consistent with the toxicity of the ipilimumab-nivolumab combination and that of chemotherapy, which is nevertheless limited due to its short duration (2 cycles).
New algorithm for frontline treatment
The level of PD-L1 expression is the only prospectively validated predictive biomarker for anti-PD(L)-1 with for tumours expressing high PD-L1 expression (≥50%), a possible choice between pembrolizumab monotherapy or a combination of chemotherapy and pembrolizumab. In the absence of a controlled trial, examination of the progression-free survival (PFS) and survival curves from the Keynote 024 (monotherapy) trial8 and the Keynote 189 and 407 (combination chemotherapy and immunotherapy) trials16, 18 respectively, suggests that chemotherapy reduces the incidence of early progression observed with pembrolizumab monotherapy. In contrast, the proportion of patients with prolonged disease control appears to be relatively similar, which will need to be confirmed by a longer follow up. The possibility of early disease progression on pembrolizumab monotherapy may argue in favour of combination with chemotherapy in the case of large tumour volume or aggressive disease, whereas in the case of more indolent disease, the use of monotherapy may appear justified to avoid the additional toxicity of chemotherapy and to allow the possibility of a platinum-based doublet as a second line therapy. The positioning of the ipilimumab-nivolumab combination is more difficult to define. This therapeutic option without chemotherapy or combined with a short course of chemotherapy could be offered to patients whose tumours do not express PD-L1, whereas in the case of high PD-L1 expression, it does not seem to offer any obvious advantage over pembrolizumab in terms of long-term benefit with an increased risk of toxicity.
Literature:
1 Mok TS et al.: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361(10): 954-7 2 Brahmer J et al.: Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. NEngl J Med 2015; 373(3): 123-35 3 Borghaei H et al.: Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373(17): 1627-39 4 Herbst RS et al.: Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016(10027); 387: 1540-50 5 Rittmeyer A et al.: OAK Study Group. Atezolizumab versus docetaxel in patients with previ-ously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389 (10066): 255-65 6 Garon EB et al. : Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 2019; 37(28): 2518-27 7 Brahmer JR et al.: Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36(17): 1714-68 8 Reck M et al.: Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced Non-Small-Cell Lung Cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37(7): 537-46 9 Carbone DP et al.: CheckMate 026 Investigators. First-line nivolumab in stage IV or recur-rent non-small-cell lung cancer. N Engl J Med 2017; 376(25): 2415-26 10 Mok TSK et al.: Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): arandomised, open-label, controlled, phase 3 trial. KEYNOTE-042 Investigators. Lancet 2019; 393(10183): 1819-30 11 Spigel D et al.: IMpower110: interim overall survival analysis of a phase III study of ate-zolizumab vs platinum-based chemotherapy as first-line treatment in PD-L1–selected NSCLC. Annals of Oncology 2019; 30(suppl_5): v851-934 12 Rizvi NA et al.: Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020; 6(5): 661-74 13 Hellmann MD et al.: Nivolumab plus ipilimumab in lung cancer with a high tumor muta-tional burden. N Engl J Med 2018; 378(22): 2093-104 14 Hellmann MD et al.: Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019; 381(21): 2020-31 15 Ramalingam S et al.: Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1. J Clin Oncol 2020; 38(suppl): abstr 9500 16 Gandhi L et al.: Pembrolizumab plus chemotherapy in metastatic non-small-cell lung can-cer. N Engl J Med 2018; 378(22): 2078-92 17 Socinski MA et al.: Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378(24): 2288-301 18 Paz-Ares L et al.: Pembrolizumab plus chemotherapy for squamous non-small cell lung cancer. N Engl J Med 2018; 379(21): 2040-51 19 West H et al.: Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(7): 924-37 20 Jotte R et al.: Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial [published online ahead of print, 2020 Apr 14]. J Thorac Oncol 2020; S1556-0864(20)30292-6 21 Papadimitrakopoulou VA et al.: IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol 2018; 13: OA 05-07 22 Reck M et al. Nivolumab + ipilimumab + 2 cycles of platinum-doublet chemotherapy vs 4 cycles chemo as first-line treatment for stage IV/recurrent non-small cell lung cancer: CheckMate 9LA. J Clin Oncol 2020; 38(suppl): abstr. 9501
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...