Numerous Hot Topics in Lymphoproliferative Disorders
Authors:
Dr. med. Noémie Lang1,2
Prof. Dr. med. Urban Novak3
1Department of Oncology
Hôpitaux Universitaires de Genève
Geneva
2University of Geneva
3Department of Medical Oncology
Inselspital, Bern University Hospital,
University of Bern, Switzerland
Correspondence
E-Mail: noemie.lang@hcuge.ch
In the field of lymphoma, plenty of exciting abstracts were presented at the 2022 meeting of the American Society of Hematology (ASH). Hereby, we will focus on a few highlights, e.g. in entities like classical Hodgkin lymphoma or primary nervous system lymphoma.
Keypoints
-
Frontline ibrutinib-containing regimen as induction and maintenance demonstrated superior efficacy compared to standard immuno-chemotherapy in young and fit mantle cell lymphoma patients.
-
Pirtobrutinib, a novel Bruton tyrosine kinase inhibitor (BTKi) active against resistant clones, demonstrated efficacy across several lymphoid malignancies.
-
High-dose immuno-chemotherapy and autologous stem cell transplantation remains standard of care for primary central nervous system lymphoma.
-
ABC DLBCL subtype may benefit from the addition of bortezomib to frontline R-CHOP regimen.
MCL: TRIANGLE phase III study
Mantle cell lymphoma (MCL) is a rare lymphoma subtype, representing approximatively 5–10% of non-Hodgkin lymphomas and usually presenting with advanced stage and extranodal involvement, particularly the gastro-intestinal tract (80%). Except very localized stages, MCL remains an incurable lymphoma with 3-year OS range of 75–81% in young patients.1–4
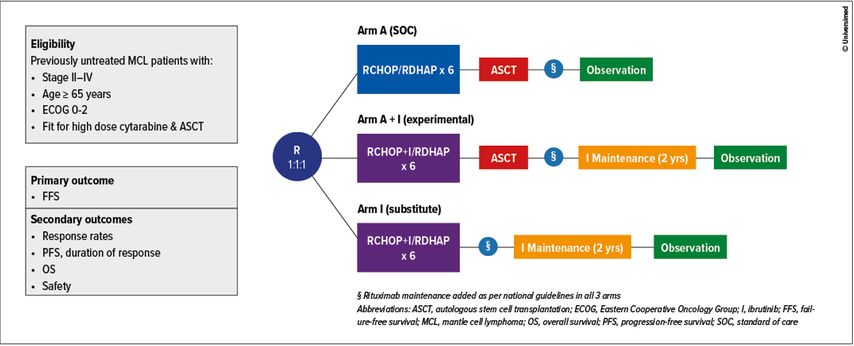
An important practice-changing abstract has been presented by Prof. M. Dreyling (abstract #1) on behalf of the European MCL Network at the plenary session, reporting the results from the international phase III TRIANGLE trial (Figure 1). As a reminder, this study aimed to challenge current frontline standard of care (SOC), referred as induction with high-dose cytarabine-containing immunochemotherapy (ICT) followed by autologous stem cell transplantation (ASCT) and rituximab maintenance, by adding ibrutinib (I) – a Bruton tyrosine kinase inhibitor (BTKi) – to the induction ICT and maintenance regimen in young transplant-eligible MCL patients (≤65 years) (Figure 1).
Initiated in 2016, this three-arm study evaluates the superiority of either alternating RCHOP + ibrutinib / RDHAP followed by ASCT and I maintenance (“experimental” arm or A+I) or alternating I-RCHOP / RDHAP without ASCT followed by I maintenance (“substitute” arm or I) in comparison to alternating I-RCHOP / RDHAP followed by ASCT (“SOC” arm or A). Alternating ICT was administered for a total of 6 cycles, I maintenance was administered continuously for up to 2 years (Figure 1). An amendment to the trial was performed to allow rituximab maintenance given according to national clinical routine after the previous demonstration of an OS benefit.3,5
A total of 870 previously untreated MCL patients suitable for high-dose cytarabine ICT and ASCT presenting with advanced stage II-IV MCL have been randomized 1:1:1 to the trial arms: A (n=288), A+I (n=292), and I (n=290). Median age was 57 years (range 27–68) with a male predominance (76%). As expected, majority of patients had stage IV disease (87%) with low, intermediate and high risk MIPI (MCL International Prognostic Index) 58%, 27%, 15%, respectively. Overall, proportion of patients that received rituximab maintenance were 58% (n=168), 57% (n=165), 54% (n=158) respectively for A, A+I and I. At the end of the induction therapy, overall response rates (ORR) and complete remission (CR) rates for evaluable patients were 94% and 36% for A versus 98% and 45% for combined I and A+I arms. With a median follow-up of 31 months, the study met its primary aim demonstrating a superior 3-year FFS for A+I vs A (88% vs 72%, p=0.0008, HR 0.52) with no superior advantage of A vs I (3-year failure-free survival (FFS) of 72% versus 86%, p=0.9979, HR 1.77). Comparison analysis between the two ibrutinib-containing regimens (A+I and I) is not yet mature.
These results were maintained across all subgroups, including high P53 expression and high-risk biology. There was no difference in efficacy by rituximab maintenance groups. Although not yet mature, respective 3-year overall survival (OS) rates were 86%, 91% and 92% for A, A+I and I. Interestingly, there were no meaningful differences in the occurrence of grade 3–5 adverse events (AEs) with the addition of I to induction ICT. However, there were substantially more grade 3–5 AEs in A+I as compared to A and I during maintenance.
In conclusion, the addition of ibrutinib during induction and as maintenance with or without ASCT demonstrated superior efficacy compared to SOC with an acceptable safety profile. Results of this impressive international effort clearly support the use of ibrutinib in first-line therapy of young MCL patients. Longer follow-up is needed to clarify if ASCT consolidation will remain a cornerstone after frontline I-containing regimen.
CLL/SLL, WM, MCL and RT: BRUIN phase I/II study
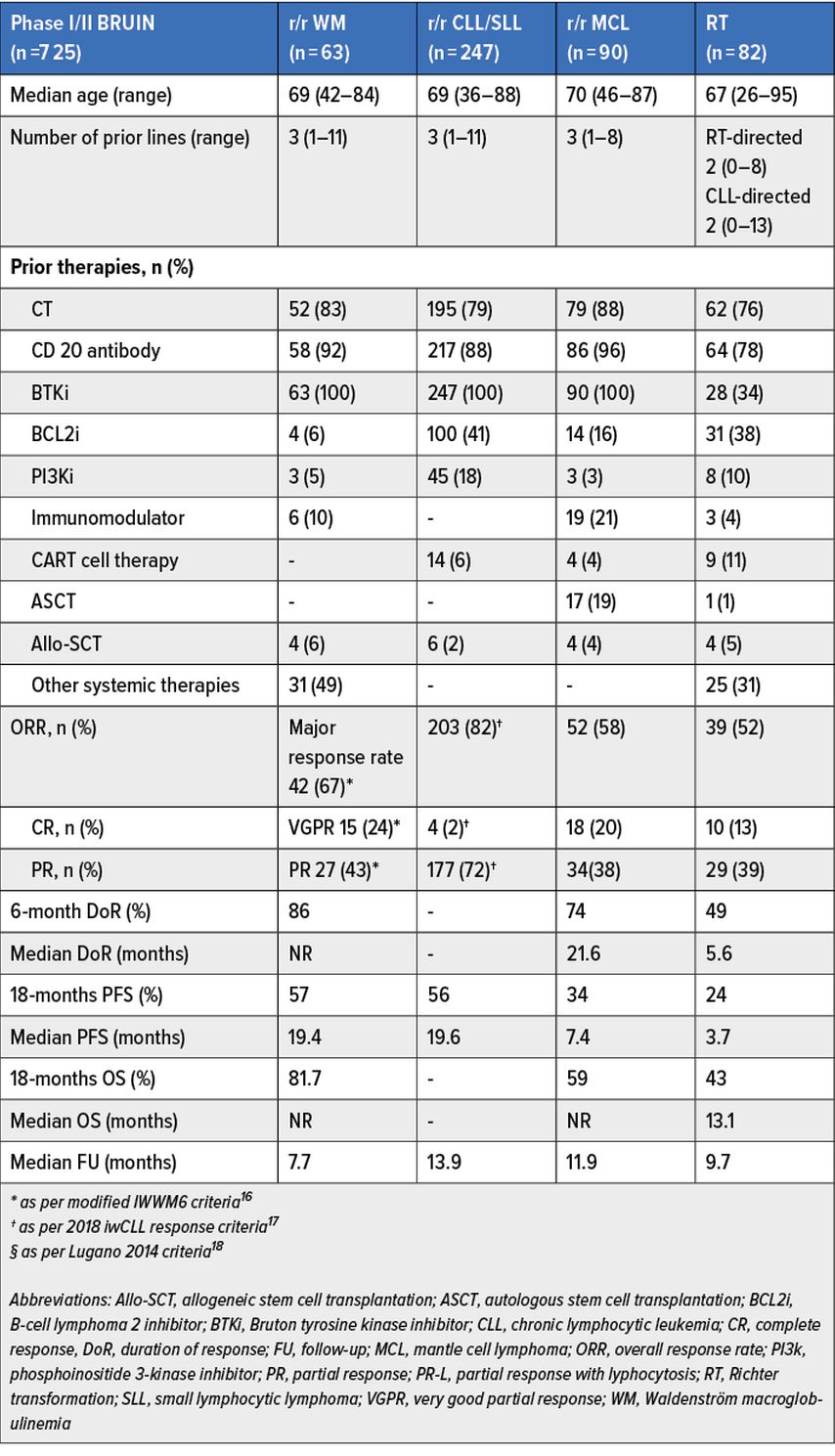
First and second generation covalent BTKi are yet routinely used in chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia (SLL)6–11 (abstract #LBA-6) and Waldenström macroglobulinemia (WM).12–13 However, their effectiveness might be hampered by intolerance and / or development of resistance.14 We will briefly discuss the results for patients with WM (n=78) (abstract #229), CLL/SLL (n=275) (abstract #961), MCL (n=90) (abstract #4218) and Richter transformation (RT) (n=57) (abstract # 347) as part of the multicenter phase I/II BRUIN study (n=725).
This trial evaluated the safety and efficacy of daily 200mg pirtobrutinib, a novel non-covalent (reversible) highly selective BTKi that equally inhibits both wildtype (WT) and C481-mutant malignant clones, across different B-malignancies.15 Main patients’ characteristics, efficacy and survival results of pirtobrutinib are summarized in Table 1.16–18 For more detailed information, we invite readers to refer to the original abstracts.
Tab. 1: Results from WM, CLL and Richter transformation (RT) expansion cohorts of the multicenter phase 1/2 BRUIN study. Modified from Abstr. #229, #961, #4218, #347
Taking altogether, updated results of the BRUIN phase I/II trial confirmed that pirtobrutinib is a well-tolerated BTKi, with low drug-related toxicity discontinuation rates (2%); most relevant reported treatment-related adverse events (TEAEs) were fatigue (26%), diarrhea (22%) while grade ≥3 TEAEs include neutropenia (20%), hypertension (3%), hemorrhage (2%), and atrial fibrillation/flutter (1%) (abstract #961). Furthermore, pirtobrutinib demonstrated promising and durable efficacy in heavily pretreated WM, CLL/SLL, MCL and devastating RT populations, regardless of prior therapy and poor-risk features.
With multiple commercially available BTKi nowadays and in the light of other abstracts presented on the role of novel genetic prognostic markers (abstracts #345 and #346) and predictive value of minimal residual disease (MRD) in CLL (abstract #92), it would be very interesting to follow how sequencing and combination approaches will evolve in the near future. As per analogy to chronic myeloid leukemia (CML) and solid tumors, monitoring acquired mutations and choosing best available drug to avoid and treat-resistant malignant clones will certainly become prerequisites.
cHL: HD21 phase III study
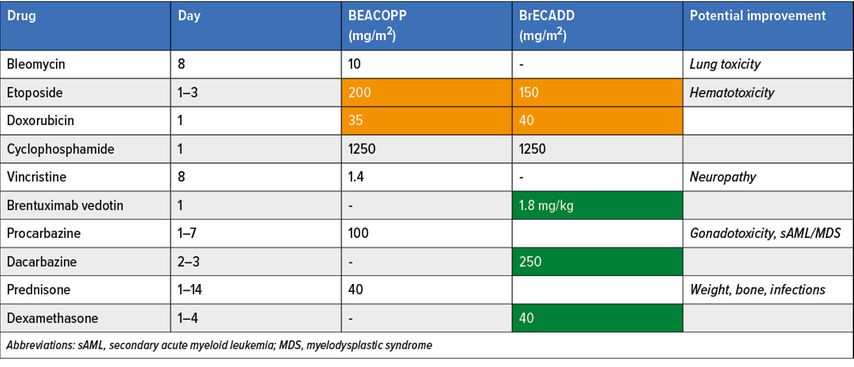
Classical Hodgkin lymphoma (cHL) patients achieve remarkably high curative rates after frontline PET-adapted BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone)-based approaches. However, BEACOPP treatment’s burden still rises concerns among the lymphoma community limiting its worldwide implementation.19–21 On behalf of the co-authors, Prof P. Borchmann reported the first results of the HD21 phase III frontline PET-guided study conducted in advanced-stage cHL patients comparing standard escBEACOPP to a modified regimen named BrECADD (brentuximab vedotin, etoposide, cyclophosphamide, doxorubicine, dacarbazine and dexamethasone) (abstract #317). Differences in regimens are provided in Table 2. The study aims to demonstrate superiority in treatment related morbidity (TRMB) and non-inferiority in efficacy of BrECADD over escBEACOPP. Overall, 1470 cHL patients were recruited (escBEACOPP, n=732; BrECADD, n=732), with a male predominance (56%) harboring stage IIB (16% both arms), stage III (39% BrECADD; 40% BEACOPP) and stage IV (45% both arms) disease. Of note, one fifth of patients were ≥45 years and other parameters such as the presence of bulk or extranodal disease were well-balanced between both arms.
Tab. 2: HD-21 phase III study comparator arms. Modified from Borchmann P et al.: ASH 2022; Abstr. #317
The study clearly met its first primary endpoint with a significant and clinically relevant reduction in TRMB favoring BreCADD regimen; red cell and platelets transfusion rates were cut by half: 24% vs 53% and 17% vs 34%, respectively. Additionally, grade 2/3 polyneuropathy was also significantly decreased (7% vs 16%). Similarly, FSH levels at one year are normalized in the BreCADD group for both male and female. With almost 3 years follow-up, progression-free survival (PFS; 94%) and OS rate results (99%) of the entire study cohort seem comparable to other frontline BEACOPP-containing studies.
PCNSL: MATRix/IELSG-43 phase III study
Another important late-breaking abstract was presented by Prof. G. Illerhaus reporting the first results of MATRix/ IELSG-43 international phase III study. Current therapeutic approach for primary central nervous system lymphoma (PCNSL) fit patients (up to 70 years) consists in 4 cycles of high-dose methotrexate-based induction, such as MATRix regimen (rituximab 375mg/m2/d days 0 & 5; methotrexate 3.5g/m2 day 1; cytarabine 2×2g/m2/d days 2 & 3; thiotepa 30mg/m2 day 4, every 21 days), followed by consolidative high-dose chemotherapy (HDCT, BCNU 400mg/m2 (day -6) and thiotepa 2x5mg/kg/d days -5 & -4) and ASCT in responding patients.22, 23
The present study evaluated the ability to replace HDCT/ASCT by 2 cycles of non-myeloablative R-DeVIC regimen (rituximab 375mg/m2 day 0; dexamethasone 40mg/d days 1 to 3; etoposide 100mg/m2/d days 1 to 3; ifosfamide 1500mg/m2/d days 1 to 3; carboplatin 300mg/m2 day 1). Stem cell harvest was conducted after the 2nd cycle of MATRix for responders. A total of 368 patients were registered, 75% (n=260) were able to complete MATRix induction and further randomly assigned to HDCT/ASCT (n=114) or R-DeVIC (n=115). Median age was 59 years (range 21–70) with 22.3% of patients being ≥65 years.
With a median follow-up of 44 months, ORR after MATRix induction was 69% (n=239) with 27% CR and 52% PR for the whole cohort. At the end of consolidation, CR rate were considerably increased in both arms (65% for R-DeVIC and 68% for HDCT/ASCT, p=0.71). However, despite similar remission rate, 3-year PFS rate was significantly higher in patients receiving HDCT/ASCT rather than R-DeVIC (79% vs 53%, HR: 0.42; p=0.0003); also confirmed by an improvement in 3-year OS rate (86% vs 71%, HR: 0.47; p=0.01). No significant differences in tolerability, treatment-related complications or neurocognitive functions were shown between arms.
In summary, this study confirms that HDCT/ASCT consolidation remains the best therapeutic strategy in suitable PCNSL patients, achieving significantly better outcomes than non-myeloablative ICT.
Aggressive B-cell lymphoma: REMoDL-B phase III study
Five-year survival results of UK/Swiss REMoDL-B phase III trial assessing the adjunction of bortezomib to frontline in DLBCL patients stratified by cell of origin (COO) determined by gene expression (GE) profile, were presented by Prof. AJ. Davies.
As a reminder, in this study, 801 patients with available COO were randomized to receive either R-CHOP or BR-CHOP and show no difference in PFS or OS by treatment arm noted after 30-month follow-up.24 COO classification has been retrospectively reassessed using a GE-based classifier and identified a high-grade group (MHG) with distinct molecular features. It represents 10% of all participants (n=83), integrated to the present analysis additionally to GCB (germinal center B-cell; 59%, n=469) and ABC (activated B-cell; 31%, n=249) COO subgroups.
At a median follow up of 64 months, no overall benefit of bortezomib on PFS or OS was shown for the entire cohort, however, a 15% and 26% significant benefit in 60-month PFS and OS with RB-CHOP was shown for ABC (HR: 0.65, p=0.041) and MHG (HR: 0.46; p=0.011) subtypes, respectively. Improvement in 60-months OS (13%) was only seen for the ABC subtype (HR: 0.58; p=0.032); MHG (HR: 0.62, p=0.16). In conclusion, ABC and MHG subtypes determined by GE profiling may benefit in the long term from the addition of bortezomib in the frontline setting.
Efficacy of BiTCEs in indolent and aggressive lymphomas
Finally, bispecific T-cell engagers agents (BiTCEs) are powerful new immunotherapeutic agents designed to recognize and bind two different antigens and improve tumor cell eradication by bringing T cells directly in contact with tumor cells. An impressive number of abstracts were presented at this meeting. They are too numerous to be summarized herby in detail, but overall confirm their durable efficacy and favorable tolerance profile (low CRS and ICANS rates) in indolent and aggressive B-cell lymphomas:
-
mosunetuzumab in r/r follicular lymphoma (FL) (abstract #610, P1588) and newly diagnosed diffuse large B cell lymphoma (DLBCL) (abstract #738);
-
odronextamab in r/r FL and r/r DLBCL (abstracts #949, and #444);
-
glofitamab in r/r MCL (abstract #74), r/r DLBCL (abstracts #441, P2983) and
-
epcoritamab in r/r DLBCL (P3580, P4251) and RT (abstract #348).
BiTCEs are valuable therapeutic options in the r/r setting and also offer multiple combination opportunities across all B-cell lymphoid malignancies:
-
mosunetuzumab (P1637, P1630, P2890);
-
glofitamab (abstract #737, P1630, P1659, P4259, P1360);
-
epcoritamab (abstracts #609, #611 and #443, P4206).
It will be challenging to put these new options in the treatment algorithm of the various lymphoma entities.
References:
1 Martin P et al.: J Clin Oncol 2022; 20; 41(3): 541-54 2 Hermine O et al.: Lancet 2016; 388: 565-75 3 Le Gouill S et al.: N Engl J Med 2017; 377: 1250-60 4 Eskelund CW et al.: Br J Haematol 2016; 175: 410-8 5 Kluin-Nelemans HC et al.: N Engl J Med 2012; 367: 520-31 6 Shanafelt TD et al.: Blood 2022; 140: 112-20 7 Byrd JC et al.: N Engl J Med 2014; 371: 213-23 8 Sharman JP et al.: Lancet 2020; 395: 1278-91 9 Ghia P et al.: J Clin Oncol 2020; 38: 2849-61 10 Hillmen P et al.: Future Oncol 2020; 16: 517-23 11 Tam CS et al.: Lancet Oncol 2022; 23: 1031-43 12 Buske C et al.: J Clin Oncol 2022; 40: 52-62 13 Tam CS et al.: Blood 2020; 136: 2038-50 14 Skånland SS, Mato AR: Blood Advances 2021; 5: 334-43 15 Mato AR et al.: Lancet 2021; 397: 892-901 16 Owen RG et al.: Br J Haematology 2013; 160: 171-6 17 Hallek M et al.: Blood 2018; 131: 2745-60 18 Cheson BD et al.: J Clin Oncol 2014; 32: 3059-67 19 Borchmann DP et al.: Lancet 2017; 390(10114): 2790-802 20 Kreissl S et al.: PET-guided eBEACOPP treatment of advanced-stage Hodgkin lymphoma (HD18): follow-up analysis of an international, open-label, randomised, phase 3 trial. The Lancet Haematology 2021; 8: e398–409 21 Borchmann P et al.: Lancet Oncol 2021; 22: 223-34 22 Ferreri AJM et al.: Lancet Haematol 2016; 3:e217-27 23 Ferreri AJM et al.: Leukemia 2022; 36: 1870-8 24 Davies A et al.: Lancet Oncol 2019; 20: 649-62
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...