SBRT for localized prostate cancer – the new standard of care in Switzerland?
Authors:
PD Dr. med. Mohamed Shelan
Prof. Dr. med. Daniel M. Aebersold
Department of Radiation Oncology
Inselspital, University Hospital Bern
Correspondence:
E-Mail: mohamed.shelan@insel.ch
Vielen Dank für Ihr Interesse!
Einige Inhalte sind aufgrund rechtlicher Bestimmungen nur für registrierte Nutzer bzw. medizinisches Fachpersonal zugänglich.
Sie sind bereits registriert?
Loggen Sie sich mit Ihrem Universimed-Benutzerkonto ein:
Sie sind noch nicht registriert?
Registrieren Sie sich jetzt kostenlos auf universimed.com und erhalten Sie Zugang zu allen Artikeln, bewerten Sie Inhalte und speichern Sie interessante Beiträge in Ihrem persönlichen Bereich
zum späteren Lesen. Ihre Registrierung ist für alle Unversimed-Portale gültig. (inkl. allgemeineplus.at & med-Diplom.at)
High-precision stereotactic body radiation therapy (SBRT), delivering high single doses per fraction, allows prostate cancer treatment to be completed within just a few days. Randomized studies have shown excellent cure rates with minimal side effects, positioning SBRT as a standard treatment option.
Keypoints
-
Level I evidence shows no significant survival differences between RP and RT exists.
-
For intermediate-risk prostate cancer (IR-PC), SBRT offers excellent oncological outcomes without a significant increase in toxicity in LR and IR-PC.
-
For high-risk PC (HR-PC), the evidence for SBRT is increasing, and clinical trials are awaited.
Prostate cancer is the most common type of cancer in men. Due to the increased awareness and prostate-specific antigen (PSA) screening, most of the cases are diagnosed at an early stage, resulting in very good chances of a cure. For localized prostate cancer, several treatment options are available, including active surveillance (AS), surgery, or radiation therapy. Radical prostatectomy (RP) and radiation therapy (RT) are the two established curative treatment options for localized prostate cancer (PC). Both approaches offer comparable chances of cure, halving the incidence of metastases compared to AS and achieving excellent local control.1 Each option has specific advantages and disadvantages due to their distinct side effect profiles and timelines.
Patient-reported outcomes (PROs) 12 years after the treatment of localized PC from the prominent randomized PROTECT study, which compared RT with RP, have been published. Patients in the RT arm had lower rates of urinary incontinence (3–8%) and a higher proportion of patients with satisfactory erections (27%). Conversely, patients in the RP arm had a higher incidence of urinary incontinence (18–24%), and only 18% of them had satisfactory erections.2 Chronic side effects (SE) in the rectum were rarely observed due to modern radiation techniques. Rectal SE of grade 2 or higher occurred about 7% more frequently after RT than after RP. Higher-grade toxicity was very rare (<1%), and quality-of-life reports were excellent.
A disadvantage of the previous, conventionally fractionated external RT (single dose of approximately 2 Gray [Gy]; total dose of up to 80 Gy) is the large number of radiation sessions required for curative RT, which can last up to two months. To counter this time burden, shortened treatment schedules with slightly increased single doses have been investigated since the early 2000s. Results from multiple randomized studies have shown that these moderately hypofractionated regimens, with single doses of 2.5–3 Gy over four weeks, are not inferior to standard regimens in terms of biochemical control and tolerability.3–5
Image-guided SBRT is a more modern treatment option. Thanks to precise positioning technologies, it enables an increased single dose per session, known as ultra-hypofractionation. According to guidelines from leading European and American oncology societies (ESTRO, ASTRO, and ASCO), ultra-hypofractionated RT is defined as radiation with a daily dose of 5.0Gy or more.6
Prostate SBRT in low-risk and intermediate-risk PC
In addition to numerous retrospective and prospective series, two randomized trials compared the shortened radiation course (5–7 fractions) to the other longer fractionation courses (20–39 fractions). The HYPO-RT-PC study randomized men with PC at intermediate and high risk to receive either 42.7 Gy in seven fractions over three days per week or conventional fractionated RT (78 Gy in 39 fractions over five days per week). After five years of follow-up, disease-free survival was identical in both groups at 84%.7 Acute side effects occurred slightly less frequently in the conventional fractionation arm than in the SBRT arm, while late toxicity was similar in both treatment groups.7 A follow-up publication on long-term quality of life revealed that SBRT was as well-tolerated as conventional treatment up to six years after treatment.8
In 2019, the results of the randomized PACE-B trial, which investigated the toxicity of SBRT in PC, were published. In this study, men with low-risk or intermediate-risk PC (Gleason 7a only) received either traditional RT (39 or 20 fractions) or ultra-hypofractionated SBRT (36.25 Gy in five fractions over 1–2 weeks). In 2022, the two-year toxicity data was published in Lancet Oncology, and it was comparable for SBRT with five fractions and conventional radiation regimens. Prostate SBRT was considered safe and had low rates of side effects.9
The PACE-A study is the first study to compare state-of-the-art treatment options for prostate cancer in both arms comparing radical prostatectomy and SBRT delivered in 5 fractions, making it highly valuable and relevant for clinical practice. A total of 123 men were randomized between August 2012 and February 2022 (60 underwent prostatectomy, and 63 received SBRT, all completed as planned in 5 fractions). The median age was 65.5 years, and the median PSA level was 7.9ng/ml. A total of 92% had intermediate-risk disease, as assessed by the National Comprehensive Cancer Network (NCCN) classification system.
PROs were collected at baseline, weeks 4 and 12, and months 6, 9, 12, and 24 using questionnaires. The co-primary endpoint was the number of incontinence pads used per day. After two years, 16 of 32 (50%) of the operated patients and 3 of 46 (6.5%) of the irradiated patients used one or more pads daily (p<0.001). The post-radiation therapy rate corresponded to the normal value for this age group, indicating that radiation therapy had virtually no impact on continence. This makes a significant difference in patients’ quality of life. Incontinence is stigmatized and remains a major taboo subject, often leading affected individuals to withdraw socially, with loneliness, low self-esteem, and even depression as potential consequences. Maintaining continence is therefore a crucial factor in therapy decisions for many prostate cancer patients.
Preserving sexual function is equally important for quality of life, and in this regard, patients in the study also benefited significantly from radiation therapy. Operated men rated this aspect of their lives much lower than irradiated men (median 19 points vs. 62.5 points). The proportion of men reporting moderate to severe sexual problems was 10 of 30 (33%) in the prostatectomy group and 8 of 45 (18%) in the radiation therapy group.
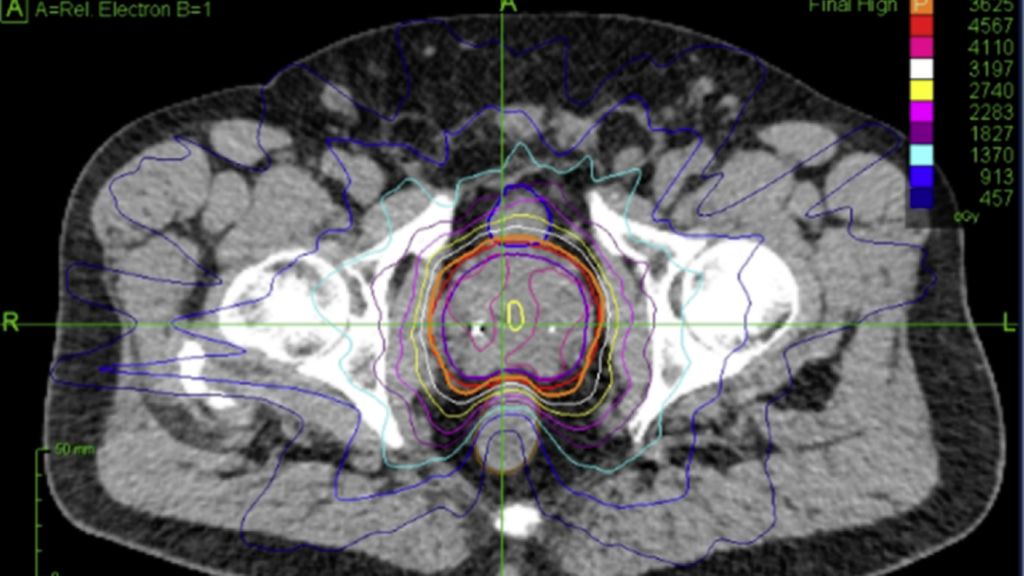
The only advantage observed for surgery was in bowel function, also assessed using the EPIC score. Operated patients reported a median score of 100, compared to 87.5 for irradiated patients, with the difference being statistically significant. However, this difference is «overall small in clinical reality.» Moderate or severe impairments were not observed in any of the 31 prostatectomy patients and only one of the 48 irradiated patients (2.1%). Fecal incontinence rates did not differ between the groups after 24 months (Tab. 1 & Fig. 1).
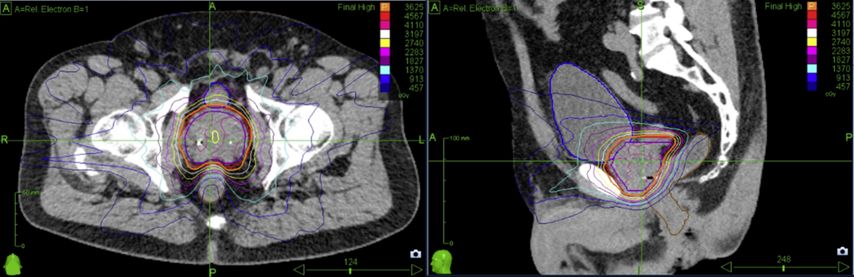
Fig. 1: Dose distribution for prostate cancer patients treated on robotic SBRT platform (Cyberknife)
Prostate SBRT in high-risk PC
Due to the limited number of high-risk patients included in prospective studies, SBRT for HR-PC remains under investigation. Initial reports, however, indicate reasonable oncological outcomes and manageable toxicity. Biochemical control rates range from 82% to 100% at 2 years and 56% to 100% at 3 years.11 Acute grade ≥2 genitourinary (GU) toxicity is reported at 12% to 46.7% with pelvic elective nodal irradiation (ENI) and 0% to 89% in prostate-only studies, while late GU toxicity ranges from 7% to 60%.11 A recent meta-analysis of 417 patients receiving SBRT to the pelvic nodes showed acute grade ≥2 GU and gastrointestinal (GI) toxicity rates of 29% and 8%, respectively, with late GU and GI toxicity at 29% and 13%.12 While SBRT offers outcomes comparable to conventional RT, it is not yet the standard of care for HR-PC due to the lack of Level 1 evidence. SBRT treatment may be considered for selected patients at specialized centers with access to high-precision radiotherapy; however, further clinical trials are still needed to clarify its role.
Conclusion
The strongest evidence supports SBRT as an effective alternative to both moderately hypofractionated RT and RP for localized prostate cancer. Multiple studies have shown high biochemical control rates and low late toxicity. These findings mark a significant shift in prostate cancer treatment, offering a non-invasive, outpatient curative option that minimizes patient burden.
Conflict of Interest Statement
The authors have declared no potential conflicts of interest.
Literatur:
1 Hamdy FC et al.: Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2023; 388(17): 1547-58 2 Donovan JL et al.: Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016; 375(15): 1425-37 3 Lee WR et al.: Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 2016; 34(20): 2325-32 4 Dearnaley D et al.: Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016; 17(8): 1047-60 5 Catton CN et al.: Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017; 35(17): 1884-90 6 Morgan SC et al.: Hypofractionated radiation therapy for localized prostate cancer: executive summary of an ASTRO, ASCO and AUA evidence-based guideline. J Urol 2019; 201(3): 528-34 7 Widmark A et al.: Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019; 394(10196): 385-95 8 Fransson P et al.: Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol 2021; 22(2): 235-45 9 van As N et al.: Phase 3 trial of stereotactic body radiotherapy in localized prostate cancer. N Engl J Med 2024; 391(15): 1413-25 10 van As NJ et al.: PACE-A: an international phase 3 randomised controlled trial (RCT) comparing stereotactic body radiotherapy (SBRT) to surgery for localised prostate cancer (LPCa) – primary endpoint analysis. J Clin Oncol 2023; 41(suppl 6): Abstr. #298 11 Foerster R et al.: Stereotactic body radiotherapy for high-risk prostate cancer: a systematic review. Cancers (Basel) 2021; 13(4): 759 12 Mohamad O et al.: Safety of ultrahypofractionated pelvic nodal irradiation in the definitive management of prostate cancer: systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2024; 118(4): 998-1010
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...