Surgery or RT in NSCLC N2 Stage III?
Authors:
Dr. med. Fabio Dennstädt
Klinik für Radio-Onkologie
Dr. med. Pawel Leskow
Klink für Thoraxchirurgie
Prof. Dr. med. Paul Martin Putora
Klinik für Radio-Onkologie
Kantonsspital St. Gallen
E-Mail: fabio.dennstaedt@kssg.ch
For the management of locally advanced non-small cell lung cancer (NSCLC), lymph node extent of the disease is critical. In the setting of N2 extended stage III NSCLC, there is still an ongoing debate on whether patients should undergo surgery or be treated with radiotherapy (RT). The following article gives a brief overview about the current data.
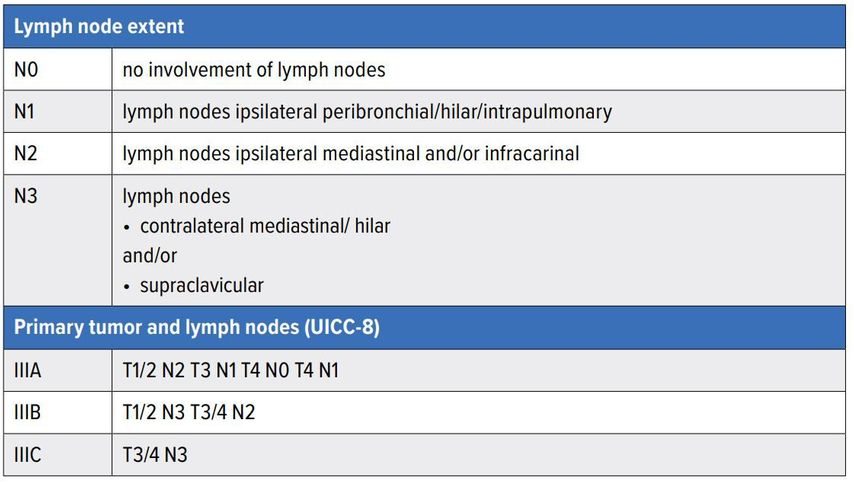
Non-small cell lung cancer (NSCLC) makes up most of lung cancer cases and is still associated with a poor prognosis. Locally advanced disease, also referred to as stage III (UICC-8) disease, represents a wide variety of disease patterns (Table1). For clinical management lymph node extent of disease is critical. Typically for N0 (no lymph nodes) and N1 disease (ipsilateral hilar lymph nodes), surgery is the standard treatment (in combination with chemotherapy). For N3 disease (contralateral mediastinal/hilar and/or supraclavicular lymph nodes) radiochemotherapy (potentially followed by immunotherapy) is the standard of care.
In the setting of ipsilateral mediastinal lymph node extent (N2) some controversy exists as to what the optimal management is. In this brief review, we will address the data and guidelines related to this question.
While the decision remains difficult...
Locally advanced N2 stage III NSCLC has been investigated in several prospective randomized trials. We will name a few here to demonstrate the findings in this setting. The trials can be viewed as belonging to two different questions – one concept investigated adding surgery to a setting of radiochemotherapy and the other investigated adding neoadjuvant radiotherapy (or radiochemotherapy) before surgery.
An important trial in this setting is the Intergroup 0139 published in 2009, which investigated two cycles of a platinum doublet chemotherapy with 45 Gy of irradiation (which is about ¾ of what would be considered a curative radiotherapy dose).1 Patients without progression were randomized to receive either surgery or continue with radiotherapy to a curative dose of 61 Gy. Overall survival (OS) was not different between the arms, while the progression-free survival (PFS) was in favor of the surgery arm. Due to a high mortality associated with pneumonectomies an exploratory analysis excluding these was performed; this selected patient group had a better OS than the radiotherapy group. Yet, it should be noted that if one was to select optimal patients from the radiotherapy arm, these would also have a better survival. Therefore, a clear benefit for adding surgery in this setting remains elusive. Another study following the same concept (with radiotherapy reaching a total dose of 65–71 Gy) was performed in three centers (Essen, Paris, Tübingen) within the ESPATÜ trial.2 This trial did not demonstrate any difference in OS or PFS.
Another way of exploring stage III NSCLC is by investigating the potential benefit of adding radiotherapy to the setting of surgery and chemotherapy. The German GLCCG 01/95 trial investigated adding radiochemotherapy with 45Gy between neoadjuvant chemotherapy and surgery. A total of 524 patients were included, but the trial did not reveal any benefit of adding neoadjuvant radiochemotherapy with the OS and PFS not being statistically different.3 Furthermore, the Swiss SAKK trial 16/00 investigated the addition of neoadjuvant radiotherapy with 44Gy (between chemotherapy and surgery), where radiotherapy was not associated with any benefit, while it did increase toxicity.4
When summarizing the results of these four trials, we can conclude that there is very poor evidence to support adding surgery to the setting of radiochemotherapy and there is poor evidence supporting the addition of radiotherapy to the setting of surgery and chemotherapy. Adding to the problem of interpretation is that surgical, radiotherapeutic and imaging techniques have evolved since the conception of these trials.
High-level evidence can sometimes be generated with meta-analyses. Attempts to identify the “best” treatment failed showing no statistically significant difference between the radiotherapeutic and surgical approach.5,6
In clinical routine, there is a trend to offer surgery to patients with a lesser extent of lymph nodes involved (as well as to younger and fitter patients).7 This leads to an inherent bias in patient selection making retrospective analyses very problematic in this setting. For this reason, individual retrospective analyses will not be addressed in this review.
In this setting of evidence equipoise we may look to guidelines for guidance. An analysis of international guidelines demonstrates that in most settings (of N2 stage III NSCLC) radiotherapy and surgery are both options. While individual guidelines prefer surgery in the setting of single station N2 disease, most consider bothtreat- ment options equivalent. With increasing lymph node extent (extending beyond a single lymph node station, or even beyond lymph node zones) the guidelines tend to recommend radiotherapy more commonly.7 Many guidelines also mention bulky lymph nodes (usually defined as having a diameter of at least 3cm), mostly advising radiotherapy in their presence.
...patients’ preferences should always be included
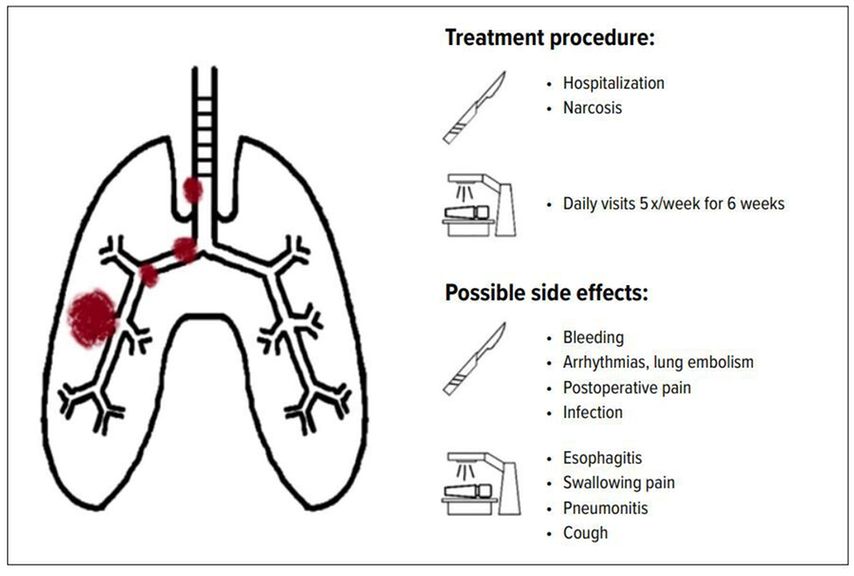
Overall, there are many situations in N2 stage III NSCLC where both surgery and radiotherapy can be offered. This provides space to include the patient in a shared decision-making approach. Especially in the setting of similar oncological outcomes the patient preference related to side effects and the patient experience (logistics of treatment) should be considered. While hospitalization and narcosis is necessary for surgery, the intervention itself takes a few hours and hospitalization is often a matter of only a few days. Radiation therapy on the other hand requires daily therapy five times a week for usually around 6 weeks.
Regarding side effects, their documentation in clinical trials is often deficient (especially in trials investigating surgery).8 Generally speaking, side effects of surgery involve risks associated with the intervention like bleeding, impaired wound healing, arrhythmias, lung embolism, postoperative uncontrolled pain, infection or perioperative mortality. Possible side effects of radiotherapy include esophagitis, swallowing pain, cough and radiation pneumonitis.
Therapeutic options should be discussed within a multidisciplinary tumor board. Patients with N2 stage III disease, if feasible, should be offered a consultation by a surgeon and a radiation oncologist and should be able to choose based on their personal preference.9 Unfortunately, a survey among Swiss radiation oncologists and thoracic surgeons has demonstrated patient preference is considered in only a small number of settings and only by a small minority of experts.10
To put it in a nutshell
For patients with stage III N2 NSCLC the treatment decision to choose surgery or radiotherapy, if both are options, should not be definitively taken in a tumor board in absence of the patient. This decision should come about through shared decision-making involving a thoracic surgeon and a radiation oncologist. Patient’s wishes and preferences should be addressed when deciding between the two oncological equivalent but conceptually very different treatment options.
Literatur:
1 Albain KS et al.: Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009; 374(9687): 379-86 2 Eberhardt WE et al.: Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015; 33(35): 4194-201 3 Thomas M et al.: Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008; 9(7): 636-48 4 Pless M et al.: Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015; 386(9998): 1049-56 5 Pottgen C et al.: Definitive radiochemotherapy versus surgery within multimodality treatment in stage III non-small cell lung cancer (NSCLC) - a cumulative meta-analysis of the randomized evidence. Oncotarget 2017; 8(25): 41670-8 6 McElnay PJ et al.: Outcome of surgery versus radiotherapy after induction treatment in patients with N2 disease: systematic review and meta-analysis of randomised trials. Thorax 2015; 70(8): 764-8 7 Putora PM et al.: International guidelines on stage III N2 nonsmall cell lung cancer: surgery or radiotherapy? ERJ Open Res 2020; 6(1): 00159-2019 8 Iseli T et al.: Adverse events reporting in stage III NSCLC trials investigating surgery and radiotherapy. ERJ Open Res 2020; 6(3): 00010-2020 9 Daly ME et al.: Management of stage III non-small-cell lung cancer: ASCO Guideline. J Clin Oncol 2021; 0(0): JCO2102528 10 Glatzer M et al.: Stage III N2 non-small cell lung cancer treatment: decision-making among surgeons and radiation oncologists. Transl Lung Cancer Res 2021; 10(4): 1960-8
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...