Which is the best front-line ALK inhibitor today for ALK+ lung cancer?
Author:
Dr. Sze Wai Chan
Medical Oncologist
Sandton Oncology, Johannesburg, South Africa
E-Mail: drchan@sandtononcology.co.za
ALK rearrangement in non-small cell lung cancer (NSCLC) was shortly discovered after EGFR mutation. There are 1st-, 2nd-, and 3rd-generation ALK inhibitors available nowadays. Which ALK inhibitors should be best initiated as first-line treatment remains a debate.
Keypoints
-
ALK inhibitors are superior to platinum-based chemotherapy as first-line treatment in ALK+ advanced NSCLC.
-
2nd/3rd-generation ALK inhibitors have better ORR, PFS and CNS control (vs.crizotinib).
-
Lorlatinib is associated with the highest PFS and intracranial CR rate in the latest 36,7 month follow-up (vs. all earlier-generation ALK inhibitors).
-
Re-biopsy for molecular profiling is important after progression on 2nd/3rd-generation ALK inhibitors as resistance mechanisms can be complex, and it may guide future treatment planning.
In NSCLC, chromosomal rearrangements involving the ALK gene loci on chromosome 2 are found in approximately 3 to 5 percent of NSCLC cases.1,2 The most common fusion partner for ALK is the EML4 gene. It is commonly associated with adenocarcinoma histology, never- or light-smokers, younger age, and high incidence of brain metastases at diagnosis.
ALK should be tested in all newly diagnosed non-squamous NSCLC. Methods for detection include next-generation sequencing (NGS), fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), and reverse transcription polymerase chain reaction (RT-PCR). Broad testing using multigene NGS assay is preferred as access to lung tissue samples is often limited at diagnosis.
ALK+ NSCLC is associated with long survival (median overall survival [mOS] = 81 months) as demonstrated in a retrospective study of patients with ALK+ stage IV NSCLC treated with an ALK inhibitor at the University of Colorado Cancer Centre between 2009 and 2017. Most of these patients received crizotinib as first-line therapy.3
Chemotherapy vs. ALK inhibitors
The 1st-generation ALK inhibitor crizotinib has demonstrated an overall response rate (ORR) of 60,8% in the study PROFILE 1001,leading to its approval.4 Subsequently, crizotinib was compared with platinum/pemetrexed in PROFILE 1014 in a treatment-naïve setting and demonstrated increase in both ORR (74% vs. 45%) and median progression-free survival (PFS), 10,9 vs. 7 months (HR: 0,45).5
In the ASCEND-4-trial, ceritinib (2nd generation ALK inhibitor) was compared with platinum/pemetrexed. Ceritinib is 20 times more potent than crizotinib and has higher intracranial efficacy. In the ASCEND-4-trial, which included 376 treatment-naïve patients, those randomly assigned to ceritinib experienced improved PFS (16,6 vs. 8,1 months; HR: 0,55), ORR (72,5% vs. 26,7%), and duration of response (23,9 vs. 11,1 months).6
2nd/3rd-generation vs. 1st-generation ALK inhibitors
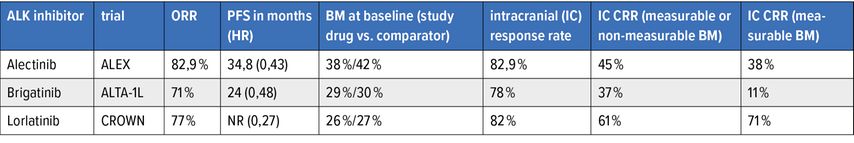
In the ALEX-, ALTA-1L- and CROWN-trials, 2nd/3rd-generation ALK inhibitors were compared with crizotinib in the treatment-naïve setting.7–11 The results are shown in Table 1. The 2nd/3rd-generation ALK inhibitors are more potent than crizotinib, have high intracranial activity and have broad coverage of ALK-resistance mutations. The study CROWN, at 36,7 months follow-up,11 has shown the longest median PFS to date amongst all ALK inhibitors (median PFS – not reached [NR] for lorlatinib vs. 9,3 months for crizotinib in the intention-to-treat population; HR: 0,27). Furthermore, lorlatinib is associated with the highest intracranial complete response rate (IC CRR) when compared with the 2nd-generation ALK inhibitors (Tab. 1).
For patients who presented without baseline brain metastases, only one patient developed brain metastases on follow-up while on lorlatinib (vs. 25 patients on crizotinib, median intracranial time-to-progression NR vs. 30,8 months; HR: 0,02).
Adverse events of ALK inhibitors
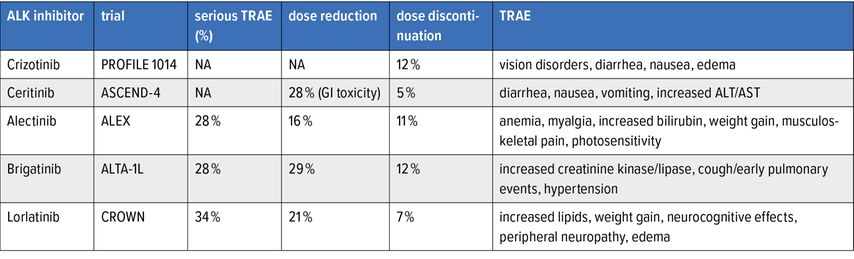
The adverse events (AE) of the currently approved ALK inhibitors are summarized in Table 2. Of note, lorlatinib has been reported to have more treatment-related adverse events (TRAE) but has a low rate of dose discontinuation.
The neurocognitive AE associated with lorlatinib are usually early (within the initial 2 months). At the post-hoc analysis of CROWN, 35% had CNS AE with lorlatinib, most were grade 1. Occurrence of CNS AE did not result in a clinically meaningful difference in patient-reported quality of life. 56% of CNS AE were resolved, of which 33% without intervention and 17% with lorlatinib dose modification. 38% were unresolved. Most AE required no intervention. Lorlatinib dose modification did not influence PFS.12
Resistance mechanisms of ALKinhibitors
Ultimately, resistance mutations developed on ALK inhibitors. Most of the resistance mutations developed after crizotinib are overcome by 2nd/3rd-generation ALK inhibitors, and re-biopsy for molecular profiling may not be necessary.
However, when 2nd-generation ALK inhibitors are used more frequently in the front-line setting, a G1202R-resistant mutation can developed in up to 40% of cases. EML4-ALK variant 3 is particularly associated with high incidence of G1202R after treatment with a 2nd-generation ALK inhibitor. The G1202R can be overcome by lorlatinib.13
When lorlatinib is used in the front-line setting, the acquired resistance mechanism is less well known. It could be in the form of ALK amplification, ALK kinase domain mutations (some of which can be overcome by the earlier generation ALK inhibitors), or bypass signaling involving off-target mutations such as EGFR/KRAS/MET/cKit/IGF-1. It can also undergo histologic/small cell transformation.
Compound mutations can develop with sequential use of 2nd- and 3rd-generation ALK inhibitors.14 Possibilities to overcome these resistance mechanisms are heavily studied in the trial space. Re-biopsy for molecular profiling should be performed after progression on 2nd/3rd-generation ALK inhibitors to select the best future treatment for the patient.
Unresolved gaps in ALK+ NSCLC
EML4-ALK variant 3 and co-existence of TP53 are poor prognostic factors in ALK+ NSCLC. It is still unknown which ALK inhibitor should be used in the front-line setting for these patients. In the early-stage setting, we await further data from the trials ALCHEMIST and ALINA to guide us to the best approach in the adjuvant setting.
Conclusion
The latest data from the CROWN-trial suggest that lorlatinib has the best PFS and CNS control to date for ALK+ advanced NSCLC. The choice for the treatment-naïve setting requires careful consideration of the burden of disease, baseline brain metastases, and side effects of treatment.
2nd/3rd-generation ALK inhibitors should be used in the first-line setting, however, the sequencing of first-line ALK inhibitors (2nd vs. 3rd) remains a debate as toxicity of lorlatinib requires a fine-tuning of management, and resistance mechanisms of lorlatinib, and future treatment options remain a concern.
Literature:
1 Koivunen JP et al.: EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008; 14(13): 4275-83 2 Gainor JF et al.: ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013; 19(15): 4273-81 3 Pacheco JM et al.: Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol 2019; 14(4): 691-700 4 Camidge DR et al.: Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012; 13(10): 1011-9 5 Solomon BJ et al.: First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371(23): 2167-77 6 Soria JC et al.: First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017; 389(10072): 917-29 7 Peters S et al.: Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377(9): 829-38 8 Mok T et al.: Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020; 31(8): 1056-64 9 Camidge DR et al.: Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018; 379(21): 2027-39 10 Camidge DR et al.: Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol 2021; 16(12): 2091-108 11 Benjamin Solomon et al.: Abstract CT223: updated efficacy and safety from the phase 3 CROWN study of first-line lorlatinib vs crizotinib in advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). Cancer Res 2022; 82(12_suppl): CT223 12 Solomon BJ et al.: Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non-small-cell lung cancer from the phase III CROWN study. J Clin Oncol 2022: JCO2102278. 13 Gainor JF et al.: Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 2016; 6(10): 1118-33 14 Recondo G et al.: Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin Cancer Res 2020; 26(1): 242-55
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...