Indications for reverse shoulder arthroplasty in 2020: What has changed?
Albany Health Campus, Albany, Australia<br>Royal Perth Hospital, Perth, Australia<br>E-Mail: blakeney@gmail.com
Ensemble Hospitalier de la Côte,<br>Clinique de Morges, Switzerland<br>E-Mail: stefan.bauer@ehc.vd.ch
Centre Orthopédique Santy,<br>Hôpital privé Jean-Mermoz, Lyon, France
Vielen Dank für Ihr Interesse!
Einige Inhalte sind aufgrund rechtlicher Bestimmungen nur für registrierte Nutzer bzw. medizinisches Fachpersonal zugänglich.
Sie sind bereits registriert?
Loggen Sie sich mit Ihrem Universimed-Benutzerkonto ein:
Sie sind noch nicht registriert?
Registrieren Sie sich jetzt kostenlos auf universimed.com und erhalten Sie Zugang zu allen Artikeln, bewerten Sie Inhalte und speichern Sie interessante Beiträge in Ihrem persönlichen Bereich
zum späteren Lesen. Ihre Registrierung ist für alle Unversimed-Portale gültig. (inkl. allgemeineplus.at & med-Diplom.at)
Paul Grammont developed the reverse total shoulder arthroplasty (RTSA) in the 1980s as a treatment for rotator cuff tear arthropathy in the elderly.23 It demonstrated excellent clinical outcomes and has thus become well-established as the treatment of choice for cuff tear arthropathy. Increasing surgeon experience with the reverse prosthesis has seen a decrease in complications.57 As a result, over the last 15 years the indications for RTSA have seen a huge expansion. The Australian National Joint Registry shows the proportion of primary total shoulder arthroplasty (TSA) cases that are reverses has increased from 42.2% in 2009 to 77.9% in 2018 (Fig.1).1 This review looks at some of the more recent evidence for the following indications: posterior glenoid deficiency with intact cuff, massive irreparable rotator cuff tears (without glenohumeral arthritis), fracture, tumour, revision surgery, and in the treatment of younger patients.
Keypoints
-
Reverse total shoulder arthroplasty (RTSA) was initially developed as a treatment for cuff tear arthropathy in the elderly.
-
Excellent clinical outcomes and survivorship have led to an explosion in its use worldwide, with expanding indications.
-
The decision to undergo RTSA is a multifactorial one looking at patient function, pain, age, medical comorbidities and social situation. Deteriorating function and increasing pain may lead to a tipping point when conservative treatments should be abandoned for surgical management.
-
Indications for use include: osteoarthritis with posterior glenoid deficiency, massive irreparable rotator cuff tears (without glenohumeral arthritis), fracture, tumour, and revision surgery.
-
There is increasing evidence for the use of RTSA in younger patients.

Fig. 1: Figure ST1 from AOA NJRR Report demonstrating increasing use of RTSA since 20081
General indications: Transition from non-operative treatment to RTSA
The decision to undergo RTSA is a multifactorial one taken by the patient in concert with the surgeon. Early in the natural history of the disease, conservative measures such as activity modification, physiotherapy, simple analgesia, and corticosteroid injections may be considered. When the patient’s pain and loss of function have progressed to the point that the risks, cost and inconvenience of undergoing surgery are outweighed by the benefits, surgery should be offered. Clinical indicators that can help in this assessment include pain scores, range of motion and patient-related outcome measures (PROMs).
High preoperative function is a known risk factor for a poorer outcome.62,64 Somerson et al. defined the time at which a patient determines that the expected improvement in pain and function following shoulder arthroplasty outweigh the risks involved as the “tipping point”.53 They used the Simple Shoulder Test (SST) scores to define a baseline score for patients electing to undergo shoulder arthroplasty, with those undergoing RTSA having a mean score of 1.5±1.8. Schoch et al. looked at a number of pre-operative indicators.47 The mean tipping point for measures of range of motion (ROM) for shoulders undergoing shoulder arthroplasty was 89° of forward flexion, 76° of abduction, 19° external rotation and internal rotation to the sacroiliac joint. The median tipping points for preoperative PROMs were as follows: American Shoulder and Elbow Surgeons (ASES) score 35.3 (SD 15.9); Constant score 36.1 (SD 14.6), SST score 3.6 (SD 2.9). Having an idea of these mean pre-operative scores for the various PROMs may help the clinician counsel the patient on the appropriate time to undergo surgery.
It is, of course, also important to consider the patient’s social situation and medical comorbidities. Absolute contraindications include deltoid and axillary nerve dysfunction and active infection.
Primary osteoarthritis with posterior glenoid deficiency
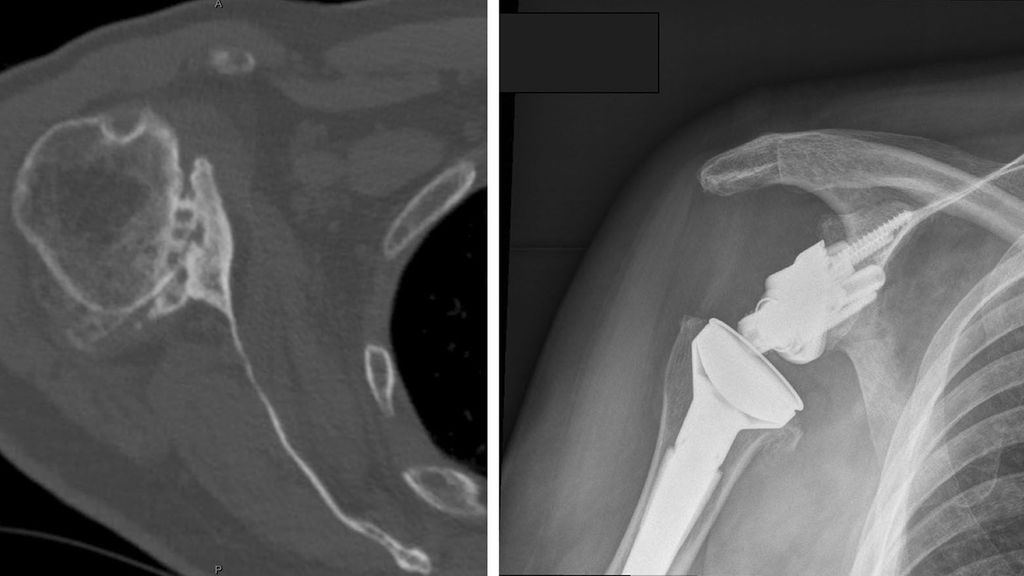
There has been an increasing trend towards the use of RTSA in patients with osteoarthritis and an intact rotator cuff, in the presence of posterior glenoid wear and/or humeral head subluxation (Fig.2). Traditionally, posterior glenoid wear has been managed with anatomic TSA and asymmetric reaming, with posterior bone graft or a posteriorly augmented glenoid for larger corrections. Walch et al., however, reported high rates of glenoid loosening following anatomic TSA in a series of 92 patients with biconcave glenoids and primary osteoarthritis.58 At a mean follow up of 77 months, glenoid loosening was observed in 20.6%, with a revision rate of 16.3%. They also reported that humeral head subluxation >80% (measured as a percentage of the humeral head posterior to the scapular axis on axial CT slice), was associated with adverse outcomes. They reported a risk of dislocation of 11% (3 of 27) in this subgroup of patients.
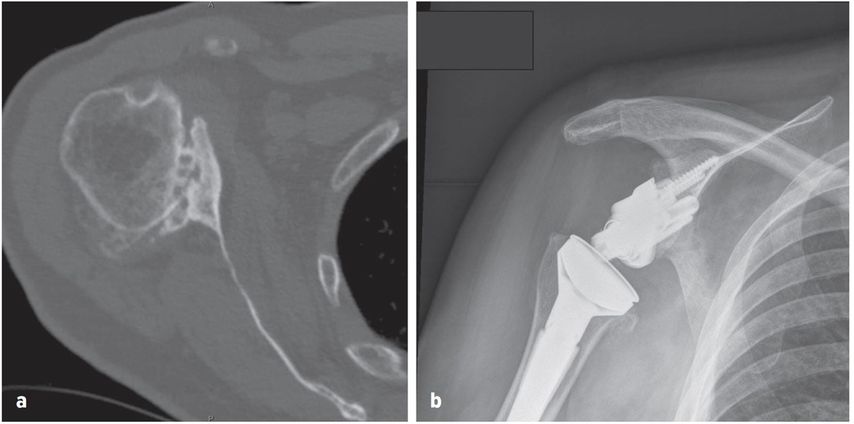
Fig. 2: Axial CT image demonstrating posterior glenoid wear (a), RTSA with posterior-augmentation on glenoid baseplate (b)
Posterior glenoid bone loss is often associated with humeral head subluxation. Ianotti et al. demonstrated that humeral head subluxation measured pre-operatively adversely affects the prognosis of both anatomic TSA and hemiarthroplasty for osteoarthritis.28 In a series of 128 shoulders, it was associated with lower ASES scores, worse pain scores, and less range of motion. In anatomic TSA, in the setting of posterior glenoid deficiency, it is likely the recurrence of humeral head subluxation post-operatively that leads to early polyethylene wear and glenoid component loosening. The high rate of complications with anatomic TSA has resulted in surgeons considering the use of RTSA, aiming to correct the posterior head subluxation with the semi-constraint inherent in the reverse’s design.
Mizuno et al. reported excellent clinical outcomes in a series of 27 patients with primary osteoarthritis and biconcave glenoids treated with RTSA, with only one failure at a mean follow-up of 54 months.40 A retrospective review of 49 shoulders with primary osteoarthritis treated with RTSA, similarly demonstrated satisfactory clinical outcomes, with only 2 failures (both due to infection, 4%), at a mean 7.7 years follow-up.10 Pre-operative glenoid morphology had no influence on outcomes.
McFarland et al. also reported on 42 consecutive RTSAs, implanted without bone-grafting, to treat glenohumeral arthritis with severe glenoid bone loss in cuff-intact shoulders.37 They demonstrated satisfactory clinical function with only one failure (due to base plate loosening, 2%) at a mean of 3 years post-surgery.
Glenoid bone loss resulting in a biconcave (B2) or severely retroverted and dysplastic (C) glenoid should be considerations for use of RTSA.
Massive, irreparable rotator cuff tears (without glenohumeral arthritis)
In the setting of a massive rotator cuff tear that is deemed to be irreparable, surgical options are limited. RTSA is a good option for certain patients. The patient should have pain and/or evidence of pseudoparalysis (persisting active shoulder elevation less than 90° of at least 3–6 months duration in the presence of free passive anterior elevation).49 This indication for surgery is generally reserved for more elderly patients. Hartzler et al. reported that age <60 was associated with poor functional outcome in the setting of RTSA for massive, irreparable cuff tear without glenohumeral arthritis.26 Tendon transfers were the mainstay of treatment for younger patients.17,22 Newer techniques such as superior capsular reconstruction have shown some early promise, but long-term follow-up is limited.14
Mulieri et al. presented a series of 72 shoulders that underwent RTSA for a massive irreparable rotator cuff tear without glenohumeral joint degenerative changes.41 They reported significant improvements at a mean 54-months follow-up, with range of motion (active forward elevation, mean 134°), functional outcomes (ASES mean 75.4), and pain (VAS 1.9), with a 20% complication rate and a 10% revision rate.
A systematic review, including 365 shoulders from 8 studies, looked at outcomes of RTSA for massive irreparable rotator cuff tears with a minimum 5-year follow-up.18 After a mean follow-up of 9.5 years (range 5–20 years), the preoperative Constant scores were significantly improved from 24 to 59 points (p=0.004). The preoperative Subjective Shoulder Value improved from 23% to 72% (p=0.049). Active anterior elevation and abduction also improved significantly. There was no significant deterioration seen in the clinical scores or range of motion up to 20 years post-surgery. However, approximately 40% of the included patients in this study had glenohumeral arthropathy, which may influence the study results. Another systematic review with shorter follow-up (24–61.4 months) looked exclusively at RTSA for massive, irreparable cuff tear without arthritis. The study included 266 shoulders from 6 studies. It demonstrated improvement in clinical outcome scores, forward flexion, external rotation, function and pain.50
A retrospective multicentre study of 42 RTSA in the setting of failed rotator cuff surgery (60% without glenohumeral arthritis) demonstrated satisfactory clinical outcomes at a mean follow-up of 50 months.4 Patients with pseudoparalysis (30 of the 42) had the best result from surgery, with active anterior elevation increased from 56° to 123° and 93% satisfaction.
Patients who present with combined loss of active elevation and active external rotation (CLEER) present a more challenging problem than simple pseudoparalysis with loss of elevation. Boileau et al. suggested a transfer of latissimus dorsi and teres major in combination with RTSA would restore external rotation as well as elevation.6 In a series of 17 patients with mean 23 months follow-up (range 12–54), they demonstrated significantly increased elevation from 74° to 149° and external rotation from –21° to 13°.6 Patient satisfaction, subjective shoulder value, and Constant scores also improved significantly. Young et al. randomised 28 patients with CLEER to RTSA with and without latissimus and teres major transfer.65 Interestingly, they found no significant difference between the two groups in range of motion or any clinical outcomes as: Activities of Daily Living and External Rotation (ADLER) score, Disabilities of the Arm, Shoulder and Hand (DASH) score, ASES score and Simple Shoulder Test (SST) score. Berglund et al. demonstrated that RTSA alone using a lateralised glenoid can restore external rotation (from −21° to 28°) in patients with CLEER.3 Lower trapezius transfer has shown promising results for the treatment of massive posterosuperior cuff tears and pseudoparalysis, and may be an alternative to latissimus dorsi and teres major transfer.16,17
These studies demonstrate that RTSA is a good option for the treatment of massive, irreparable rotator cuff tears. The ideal patient is the elderly patient with pseudoparalysis and poor prognostic indicators for cuff healing. Caution should be exercised in younger patients and consideration given to alternative surgical options, such as tendon transfers, partial rotator cuff repairs, biceps procedures or superior capsular reconstruction.
Fracture
Acute
In elderly patients, poor bone quality often results in failure of fixation for 3- and 4-part proximal humeral fractures. Poor results following hemiarthroplasty for proximal humeral fractures are also well documented, and usually relate to tuberosity migration with malunion or nonunion.5,39Numerous recent studies have demonstrated improved clinical outcomes with RTSA compared to hemiarthroplasty, for treatment of proximal humeral fractures in elderly patients.
A number of meta-analyses have compared RTSA with hemiarthroplasty for the treatment of proximal humeral fractures. They have demonstrated improvements in clinical outcome scores,36,52,61 forward flexion,21,36,52,61 abduction52 and tuberosity healing.52,61 Some studies have shown increased complications with RTSA21 whilst others have shown decreased complications61 or no difference.36
One trial randomised 62 patients over age 70 to either RTSA or hemiarthroplasty.48 They reported significantly higher mean University of California–Los Angeles (29.1 vs 21.1) and Constant scores (56.1 vs 40.0), forward elevation (120.3° vs 79.8°), and abduction (112.9° vs 78.7°) in the RTSA group. Six patients in the hemiarthroplasty group were converted to RTSA because of severe pain and limited function. However, they still had poor function (Constant score mean 21.8, range 8–51) after conversion.
A recent randomised trial compared non-operative treatment with RTSA in 59 patients aged 80 years or older with 3- and 4-part proximal humerus fractures.33 They reported no difference in clinical outcomes at 12-months between the two groups. It should be noted that this was a population of low-demand patients with multiple comorbidities. Most surgeons would only operate on a patient over 80, if they were high functioning.
A meta-analysis looked at acute versus delayed RTSA for the treatment of proximal humeral fractures in patients aged over 65.54 They found no differences in range of motion, clinical outcome scores or revision between the 2 groups, suggesting that delaying the surgery does not affect the final outcome.
A judicious option is therefore a trial of non-operative management with conversion to RTSA for those higher demand patients that are not satisfied with the results of non-operative treatment. There are, however, certain fracture patterns that when not operated upon will lead to sequelae that are problematic.
Fracture sequelae
A number of the sequelae of fractures may be treated by RTSA, including malunion, nonunion, avascular necrosis, chronic locked dislocations and post-traumatic arthritis.
Wall et al. reported their results following RTSA in 191 shoulders with a number of aetiologies, of which 33 (13.8%) had posttraumatic arthritis.60 Although all groups had good functional outcomes, patients with post-traumatic arthritis showed less improvement in Constant score, range of motion and patient satisfaction when compared to those done for cuff tear arthropathy, osteoarthritis and massive cuff tears.
Martinez et al. reported the results of 44 patients with the sequelae of proximal humerus fractures.35 There were 16 type valgus impacted malunions, 8 locked dislocations or fracture/dislocations with head collapse/necrosis, 14 surgical neck nonunions and 6 severe tuberosity malunions. The mean Constant score increased from 28 to 58, average anterior elevation increased from 40° to 100° and 86% (24 of 44 patients) were either very satisfied or satisfied. There was, however, a relatively high prosthetic dislocations rate (13.6%).
Raiss et al., in three multicentre studies, reported the results of RTSA for the sequelae of nonunions of the surgical neck,45 tuberosity malunion44 and for chronic locked dislocation of the shoulder43. The first study including 32 nonunions of surgical neck reported similar improvements in Constant score and shoulder mobility.45 Complications were much higher, with 13 complications (41%) leading to 9 revision surgical procedures (28%). The most common complication was dislocation, which occurred in 34% (11) of the patients. Increased risk of dislocation was associated with intraoperative resection of the tuberosities,45 so preservation of the tuberosities should be performed where possible.
The second study looked at 42 patients with tuberosity malunion.44 The mean Constant score increased from 20 to 55, average flexion increased from 54° to 121° and 98% were satisfied with the results. Complications occurred in 4 patients (9.5%): one dislocation, one intra-operative fracture, 1 traumatic periprosthetic fracture and 1 aseptic loosening at 13 years.
The third study included 22 patients with chronic locked dislocation of the shoulder.43 They again demonstrated similar improvements in the Constant score and range of motion, with 7 complications (32%). The complication profile differed with the most common reason for revision surgery being failure of the glenoid component (4 cases) due to bone defects on the glenoid side. Revision surgery consisted of conversion to CTA hemiarthroplasty with removal of glenoid baseplate.
Werner et al. reviewed 21 patients that underwent RTSA for chronic locked anterior dislocation by a single surgeon.63 They recommended preoperative CT scanning to assess the glenoid bone loss (mean 45% loss) and used humeral head autograft to reconstruct it. The mean Constant score improved from 5.7 to 57.2 points, with high patient satisfaction. Again, two patients (9.5%) required revision for baseplate loosening.
RTSA offers satisfactory clinical outcomes for sequelae of fracture but the surgeon needs to be aware of the specific complications: high risk of dislocation with tuberosity resection and glenoid base plate loosening with glenoid bone loss.
Tumour
Resection of the proximal humerus in the treatment of sarcomas or other neoplasms may include the tuberosities and rotator cuff to achieve appropriate margins, limiting reconstruction options. RTSA has been used increasingly and demonstrated satisfactory functional outcomes. It should be noted, however, that function in this group of patients is generally lower than for other aetiologies given the often radical resection of the tumour. Preservation of the axillary nerve and deltoid muscle function is essential for RTSA function and stability.13
Maclean et al. reported no revisions or complications at a mean follow-up of 49 months in 8 patients that underwent RTSA following oncologic resection.34 Functional outcomes were not fantastic: mean abduction 62°, Musculoskeletal Tumor Society (MTS) score 60%.
De Wilde et al. showed highly satisfactory functional outcomes (Constant score 76, mean abduction 157°) in 14 patients that underwent RTSA following oncologic resection at a mean 7.7 years follow-up.12 3 patients (21%) had major complications (2 dislocations treated with closed reduction, 1 infection requiring revision and one aseptic loosening requiring revision).
Kaa et al. reported on 16 patients that underwent RTSA following oncologic resection at a mean 44 months follow-up.29 They reported acceptable functional outcomes (mean abduction 78°, MTS 77), but with a similarly high complication rate. 4 patients (25%) underwent revision surgery (2 aseptic loosening, 1 dislocation,
1 deep infection). 2 patients (12.5%) had a peri-operative pathological fracture.
Grosel et al. reported no early complications in 13 patients that underwent reconstruction with a reverse shoulder megaprosthesis.24 They recommend RTSA in patients with life expectancy longer than 6 months, with good preoperative shoulder function and where preservation of the axillary nerve is planned.
Reconstruction in the setting of massive proximal humeral bone loss may also be performed using a humeral allograft and RTSA composite. Sanchez-Sotelo et al. reported on 8 primary and 18 revision RTSAs using humeral allograft with RTSA.46 Only 3 of the primary RTSAs were for tumour resection with 5 for severe proximal humeral bone loss after trauma. The revisions were for failed hemiarthroplasty (11), anatomic TSA (4), and RTSA (3), with the most common reason for revision being instability (10). 5-year survival was 96%, with substantial improvements in range of motion, high clinical outcome scores (ASES) and patient satisfaction.
These studies demonstrate that RTSA is a good option for restoration of satisfactory shoulder function following resection for neoplasia. Surgeons need to be aware of the high complication rates and need to consider the extent of bone and soft tissue resection, as well as patient function and life expectancy, before undertaking surgery.
Revision
A number of studies have reported on outcomes for revision of shoulder arthroplasty to RTSA.7,31,32,42,55,59 They generally report very good functional outcomes, but with complication rates as high as 47%.7 The following studies examine the results of the different indications for revision surgery.
The most common reason for revision of anatomic TSA is cuff failure or tuberosity non-union.30 A recent study by Shields and Wiater reported on outcomes of revision of anatomic TSA to RTSA exclusively for rotator cuff failure or component loosening.51 They matched 35 revision patients with 70 patients undergoing primary RTSA for cuff tear arthropathy. At a mean 50 months follow-up, pain and ASES scores were similar (p=NS). The revision group had worse subjective shoulder value scores (63 vs. 79; p=0.002), satisfaction (74% vs. 90%; p=0.03), and more complications (31% vs. 13%; p=0.02). They concluded that although function is comparable, one should expect more complications and lower satisfaction.
Hernandez et al. reported on the results of RTSA revision surgery for instability in 62 anatomic TSAs, 13 hemiarthroplasties and 7 RTSAs.27 The survivorship free from dislocation at 5 years was 79%, with the hemiarthroplasty conversion group having highest risk of instability. They also demonstrated decreased pain, improved functional outcome scores and range of motion following revision surgery. Complication rate was reasonably high, with 12 (18%) shoulders requiring repeat revision surgery.
Melis et al. reported on 37 consecutive anatomical TSA revised to RTSA for aseptic glenoid loosening/failure.38 Glenoid bone grafting was performed in 29 cases (78%). The mean Constant score increased from 24 to 55 pts and active anterior elevation from 68° to 121°. A postoperative complication occurred after revision in 11 patients (30%). 8 patients (21%) needed a subsequent reoperation because of glenoid loosening (n=3), prosthetic anterior instability (n=3), and humeral subsidence (n=2).
Wagner et al. noted a significantly higher rate of revision in RTSAs with concomitant bone grafting compared to those that did not require bone grafting (24% vs. 7% 5-year revision rate) in a series of 143 revision RTSAs.55
Revision surgery with modular, exchangeable components is often easier than with non-modular components. Crosby et al. compared 73 shoulders that required exchange of the humeral stem with 29 that had retention of a convertible-platform humeral component.11 Patients with retention had significantly shorter operative time (mean and standard deviation, 130±48 versus 195±58 minutes) and lower estimated blood loss (292±118 versus 492±334mL). The rate of intraoperative complications was significantly lower in the retention group (0% versus 15%). Patients with retention also had improved postoperative range of motion (active external rotation: 26°±23° versus 11°±23° [p=0.006]; active forward elevation: 112°±37° versus 96°±33° [p=0.055]).
These studies demonstrate that RTSA is a good option for the majority of shoulder arthroplasty revisions.
Younger patients
Greater numbers of RTSAs are being done in younger patients (less than 60–65). Surgeons have been reluctant to perform RTSAs in young patients due to concerns about longevity of the implant. Early clinical studies demonstrated a deterioration in function after 6 to 10 years.20,25 Higher rates of complications have been reported in younger patients.15 The Australian National Joint Registry (NJR) reports significantly increased revision rates in younger age groups (at 7 years: >75: 2.7%; 65–74: 3.6%; 55–64: 5.7%).1 Higher rates or revision in younger patients, however, are seen in all types of arthroplasty, not just RTSA. Wagner et al. reviewed 5494 consecutive shoulder arthroplasties (anatomic, reverse and hemiarthroplasty) performed between 1970 and 2012.56 They reported a 3% decrease in the risk for revision surgery with every 1-year increase in age. Subgroup analysis across the different types of prosthesis showed the same association with age for all.
More recent studies have reported more promising outcomes in younger age groups. Ernstbrunner et al. reported the results of 20 patients (23 shoulders) with a mean age of 57 years (range 47–59) at a mean of 11.7 years post-surgery.19 Implant survivorship was satisfactory at 91%, and there was no drop-off in clinical function. A meta-analysis of patients <65 years (mean age 56, range 21–65), that underwent RTSA for a failed previous arthroplasty or a cuff-deficient shoulder, included 8 studies with a total of 417 patients.9 The overall complication rate was 17% (range 7%–38%), with the most common complications being instability (5%) and infection (4%). The reintervention rate was 10% at 4 years, with implant revision in 7% of cases. Clinical outcome measures were highly satisfactory and the authors concluded that RTSA was a reliable procedure in patients <65 years.
Another meta-analysis of RTSA in patients <60 years similarly demonstrated that early clinical and functional outcomes were favorable, with long-term implant survivorship comparable to older patients.2
A retrospective cohort study, including 732 primary RTSA with mean 50 months follow-up (range 24–146 months), looked at outcomes across age groups.8 They found RTSA performed in patients younger than 60 years was associated with a 4.8-fold increased risk of revision (p<0.001) and lower ASES scores (62 vs. 72; p=0.002]). They suggest that these differences may be a result of both increased demand and more severe presenting diagnosis (evidenced by lower preoperative ASES scores) in younger patients.
The results of these studies should be used to guide the surgeon and the younger patient about the postoperative outcomes and expectations following RTSA. There is no doubt that it is an effective operation for the correct indications, but comes at an increased risk of revision and complications which should be considered.
References:
1 Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty Annual Report 2019, AOA, Adelaide; 2019: 1-436. https://aoanjrr.sahmri.com/annual-reports-2019 2 Bedeir YH et al.: Outcomes of reverse total shoulder arthroplasty in patients 60 years of age or younger: a systematic review. J Hand Surg 2020; 45(3): 254.e1-8 3 Berglund DD et al.: Restoration of external rotation following reverse shoulder arthroplasty without latissimus dorsi transfer. JBJS Open Access 2018; 3(2): e0054 4 Boileau P et al.: Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg 2009; 18(4): 600-6 5 Boileau P et al.: Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg 2002; 11(5): 401-12 6 Boileau P et al.: Reversed shoulder arthroplasty with modified l’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg 2010; 19(2): 20-30 7 Boileau P et al.: Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006; 15(5): 527-40 8 Brewley EE et al.: Defining the younger patient: age as a predictive factor for outcomes in shoulder arthroplasty. J Shoulder Elbow Surg 2020; 29(7S) :S1-8 9 Chelli M et al.: Reverse shoulder arthroplasty in patients aged 65 years or younger: a systematic review of the literature. JSES Open Access 2019; 3(3): 162-67 10 Collin P et al.: Mid-term results of reverse shoulder arthroplasty for glenohumeral osteoarthritis with posterior glenoid deficiency and humeral subluxation. J Shoulder Elbow Surg 2019; 28(10): 2023-30 11 Crosby LA et al.: Conversion to reverse total shoulder arthroplasty with and without humeral stem retention: the role of a convertible-platform stem. J Bone Joint Surg Am 2017; 99(9): 736-42 12 De Wilde LF et al.: Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? Clin Orthop 2011; 469(9): 2489-95 13 De Wilde LF et al.: Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orthop 2005; (430): 156-62 14 Dimock RAC et al.: Superior capsule reconstruction: what do we know? Arch Bone Jt Surg 2019; 7(1): 3-11 15 Ek ETH et al.: Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013; 22(9): 1199-208 16 Elhassan BT: Lower trapezius transfer for shoulder external rotation in patients with paralytic shoulder. J Hand Surg 2014; 39(3): 556-62 17 Elhassan BT et al.: Outcome of lower trapezius transfer to reconstruct massive irreparable posterior-superior rotator cuff tear. J Shoulder Elbow Surg 2016; 25(8): 1346-53 18 Ernstbrunner L et al.: Long-term results of reverse total shoulder arthroplasty for rotator cuff dysfunction: a systematic review of longitudinal outcomes. J Shoulder Elbow Surg 2019; 28(4): 774-81 19 Ernstbrunner L et al.: Reverse total shoulder arthroplasty for massive, irreparable rotator cuff tears before the age of 60 years: long-term results. J Bone Joint Surg Am 2017; 99(20): 1721-9 20 Favard L et al.: Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop 2011; 469(9): 2469-75 21 Ferrel JR et al.: Reverse total shoulder arthroplasty versus hemiarthroplasty for proximal humeral fractures: a systematic review. J Orthop Trauma 2015; 29(1): 60-8 22 Gerber C et al.: Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am 2006; 88(1): 113-20 23 Grammont PM, Baulot E: Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993; 16(1): 65-8 24 Grosel TW et al.: Oncologic reconstruction of the proximal humerus with a reverse total shoulder arthroplasty megaprosthesis. J Surg Oncol 2018; 118(6): 867-72 25 Guery J et al.: Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 2006; 88(8): 1742-7 26 Hartzler RU et al.: Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg 2015; 24(11): 1698-706 27 Hernandez NM et al.: Revision to reverse total shoulder arthroplasty restores stability for patients with unstable shoulder prostheses. Clin Orthop 2017; 475(11): 2716-22 28 Iannotti JP, Norris TR: Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am 2003; 85(2): 251-8 29 Kaa AKS et al.: Reverse shoulder replacement after resection of the proximal humerus for bone tumours. Bone Joint J 2013; 95-B(11): 1551-5 30 Knowles NK et al.: Revision shoulder arthroplasty: a systematic review and comparison of North American vs. European outcomes and complications. J Shoulder Elbow Surg 2020; 29(5): 1071-82 31 Levy J et al.: The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am 2007; 89(2): 292-300 32 Levy J et al.: Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br 2007; 89(2): 189-95 33 Lopiz Y et al.: Reverse shoulder arthroplasty versus nonoperative treatment for 3- or 4-part proximal humeral fractures in elderly patients: a prospective randomized controlled trial. J Shoulder Elbow Surg 2019; 28(12): 2259-71 34 Maclean S et al.: Reverse shoulder endoprosthesis for pathologic lesions of the proximal humerus: a minimum 3-year follow-up. J Shoulder Elbow Surg 2017; 26(11): 1990-4 35 Martinez AA et al.: The use of the Lima reverse shoulder arthroplasty for the treatment of fracture sequelae of the proximal humerus. J Orthop Sci Off J Jpn Orthop Assoc 2012; 17(2): 141-7 36 Mata-Fink A et al.: Reverse shoulder arthroplasty for treatment of proximal humeral fractures in older adults: a systematic review. J Shoulder Elbow Surg 2013; 22(12): 1737-48 37 McFarland EG et al.: Reverse total shoulder arthroplasty without bone-grafting for severe glenoid bone loss in patients with osteoarthritis and intact rotator cuff. J Bone Joint Surg Am 2016; 98(21): 1801-7 38 Melis B et al.: Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg 2012; 21(3): 342-9 39 Mighell MA et al.: Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg 2003; 12(6): 569-77 40 Mizuno N et al.: Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am 2013; 95(14): 1297-304 41 Mulieri P et al.: Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. JBJS 2010; 92(15): 2544-56 42 Patel DN et al.: Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21(11): 1478-83 43 Raiss P et al.: Reverse arthroplasty for patients with chronic locked dislocation of the shoulder (type 2 fracture sequela). J Shoulder Elbow Surg 2017; 26(2): 279-87 44 Raiss P et al.: Reverse shoulder arthroplasty for malunions of the proximal part of the humerus (type-4 fracture sequelae). JBJS 2016; 98(11): 893-9 45 Raiss P et al.: Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae). J Bone Joint Surg Am 2014; 96(24): 2070-6 46 Sanchez-Sotelo J et al.: Allograft-prosthetic composite reconstruction for massive proximal humeral bone loss in reverse shoulder arthroplasty. J Bone Joint Surg Am 2017; 99(24): 2069-76 47 Schoch BS et al.: Defining the tipping point for primary shoulder arthroplasty. JSES Open Access 2019; 3(4): 273-7 48 Sebastiá-Forcada E et al.: Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg 2014; 23(10): 1419-26 49 Sellers TR et al.: Massive rotator cuff tear: when to consider reverse shoulder arthroplasty. Curr Rev Musculoskelet Med 2018; 11(1): 131-40 50 Sevivas N et al.: Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elbow Surg 2017; 26(9): e265-77 51 Shields E, Wiater JM: Patient outcomes after revision of anatomic total shoulder arthroplasty to reverse shoulder arthroplasty for rotator cuff failure or component loosening: a matched cohort Study. J Am Acad Orthop Surg 2019; 27(4): e193-8 52 Shukla DR et al.: Hemiarthroplasty versus reverse shoulder arthroplasty for treatment of proximal humeral fractures: a meta-analysis. J Shoulder Elbow Surg 2016; 25(2): 330-40 53 Somerson JS et al.: The “tipping point” for 931 elective shoulder arthroplasties. J Shoulder Elbow Surg 2018; 27(9): 1614-21 54 Torchia MT et al.: Acute versus delayed reverse total shoulder arthroplasty for the treatment of proximal humeral fractures in the elderly population: a systematic review and meta-analysis. J Shoulder Elbow Surg 2019; 28(4): 765-73 55 Wagner E et al.: Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. J Bone Joint Surg Am 2015; 97(20): 1653-60 56 Wagner E et al.: The role age plays in the outcomes and complications of shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26(9): 1573-80 57 Walch G et al.: Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg 2012; 21(11): 1470-7 58 Walch G, et al.: Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg 2012; 21(11): 1526-33 59 Walker M et al.: The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21(4): 514-22 60 Wall B et al.: Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007; 89(7): 1476-85 61 Wang J et al.: Meta-analysis suggests that reverse shoulder arthroplasty in proximal humerus fractures is a better option than hemiarthroplasty in the elderly. Int Orthop 2016; 40(3): 531-9 62 Werner BC et al.: Causes of poor postoperative improvement after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25(8): e217-22 63 Werner BS et al.: Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg 2014; 23(11): 1655-61 64 Wong SE et al.: Preoperative patient-reported scores can predict postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25(6): 913-9 65 Young BL et al.: Reverse shoulder arthroplasty with and without latissimus and teres major transfer for patients with combined loss of elevation and external rotation: a prospective, randomized investigation. J Shoulder Elbow Surg 2020; 29(5): 874-81
Das könnte Sie auch interessieren:
«Auch Patienten mit Demenz profitieren von einer chirurgischen Stabilisierung»
Patienten mit Hüftfraktur und einer leichten, mittelschweren oder schweren Demenz haben ein geringeres Risiko zu sterben, wenn sie operiert werden – vor allem wenn es sich um Kopf-Hals- ...
Management periprothetischer Frakturen am Kniegelenk
Mit steigenden Versorgungszahlen der Knieendoprothetik und dem höheren Lebensalter entsprechend der Alterspyramide nimmt auch die Zahl der periprothetischen Frakturen zu und stellt die ...
Patellofemorale Instabilität
In diesem Übersichtsartikel möchten wir ein Update über die aktuelle Diagnostik und die konservativen wie auch operativen Behandlungsmöglichkeiten der patellofemoralen Instabilität geben.